Transcription
Flow Cytometry - The EssentialsPocket Guide to Flow Cytometry
Table of Contents1. Know your Cytometer2. Understanding Fluorescence and Fluorophores3. Gating Process4. Controls5. Optimization6. Panel Building7. Simple Staining Protocols8. Flow Cytometry ResourcesFlow Cytometry Basics Guide 1
1. Know your CytometerThe basis of flow cytometry is the measurement ofhow light is scattered in the forward or side directionas it passes through a particle. There are 3 essentialcomponents.Fluidics: an outer sheath fluid running at higherpressure encloses and focuses the sample creatinga single file of particles. This allows one particle at atime to be measured.Sheath fluidHydrodynamic focusing regionCells in single fileLasers: multiple lasers of different wavelengths canbe used to excite different fluorophores attachedto a particle, e.g. 488 nm and FITC, 640 nm andAPC. These can be focused together or be spatiallyseparated.Flow Cytometry Basics Guide 2
Filters and mirrors: the arrangement of filters andmirrors, known as the configuration, allowsseparation of the light and specific wavelengths tobe measured.Short pass – light below a specific wavelengthLong pass – light above a specific wavelengthBand pass – light within a specified rangeSchematic of a typical flowcytometer setupBefore you start Do you have a single cell sample at the rightconcentration?Do you know your lasers?Do you know what filters you have? Find out more atbio-rad-antibodies.com/flow-principlesFlow Cytometry Basics Guide 3
2. Understanding Fluorescence and FluorophoresFluorophores are fluorescent molecules, which canbe attached to antibodies to allow detection ofcellular markers. They accept light energy (forexample, from a laser) at a given wavelength andre-emit it at a longer wavelength as it has lowerenergy. The wavelengths of greatest absorption andemission are termed maximal absorbance andmaximal emission wavelengths.510410CD3 PE-A750CD3 PE-A750In addition to single dyes,tandem dyes comprise asmall fluorophorecovalently coupled toanother fluorophore.When the first dye isexcited its energy istransferred and activatesthe second fluorophore,which then produces thefluorescence 49d A647Data acquired on the ZE5 CellAnalyzer.Flow Cytometry Basics Guide 4
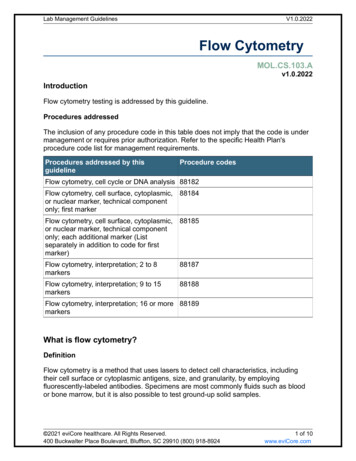
Ensure you have the right fluorophoreEnsure the fluorophore is excited by the correctlaser and the filters in the cytometer are compatiblewith the maximal emission to ensure optimal signaldetection.Table 1. Fluorophores for flow cytometry.MaximalAbsorbance, nmMaximalEmission, nmRelative BrightnessDyLight 4054004203Alexa Fluor 4054014213Pacific Blue4104551Alexa Fluor 4884955193FITC4905253DyLight 5505625764PE*496, 5465785APC6506614Alexa Fluor 6476506654DyLight 6506546734PerCP49067527027232FluorophoresAlexa Fluor 700Fluorescence ColorInfrared* PE is the same as R-phycoerythrin.APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein.Table 2. Tandem dyes for flow cytometry.MaximalAbsorbance, nmMaximalEmission, nmRelativeBrightnessPE–Alexa Fluor 647496, 5466674PE–Cy5496, 54666754906953496, 5466954FluorophoresFluorescence ColorPerCP-Cy5.5PE–Cy5.5PE–Alexa Fluor 750Infrared496, 5467794PE–Cy7Infrared496, 5467854APC–Cy7Infrared6507852* PE is the same as R-phycoerythrin.APC, allophycocyanin; PE, phycoerythrin; PerCP, peridinin chlorophyll protein.Find out more atbio-rad-antibodies.com/fluorophoresFlow Cytometry Basics Guide 5
3. Gating ProcessGates and regions can be placed aroundpopulations of cells with common characteristics toinvestigate and quantify populations you areinterested in.Forward and side scattercan give an indication ofthe size and granularity.Similar cells will grouptogether.510SSCCD3 PE-A750410310210110010010110210310410510FSCCD49d A6471.5KCountCountHistograms are singleparameter plots that giveinformation on the percentand number of cellspositive for any given marker.The positive or negative cellscan be selected to measureother 88CD3 A488Data acquired on the ZE5 CellAnalyzer.Flow Cytometry Basics Guide 6
Two–parameter dot-plots display two parameters,giving you more information, allowing you to identifysingle positive or dual positive cells.510CD3 A488CD3 A488410310210110Data acquired on the ZE5 CellAnalyzer.010010110210310410510CD69CD69 A647A647The simple principle of sequential gating can beapplied to further characterize and identify cellpopulations based on an increasing number ofmarkers.Ensure you collect enough cells, because as yourefine your populations the number of cells cansignificantly decrease.Don’t be afraid to try lots of different gatingcombinations and strategies in your analysis, yourcells may not be where you expect!Find out more atbio-rad-antibodies.com/gatingstrategiesFlow Cytometry Basics Guide 7
4. ControlsHere are the essential controls you should include inyour flow cytometry experiment:Unstained – determine where your cells are andwhat is negative.Isotype controls – determine specific surfacestaining, have you got the right isotype?Same speciesSame subclassSame fluorophore Biological controls – essential for mostexperiments, not just flow cytometry, make sure youinclude these controls.Known positiveKnown negativeUnstimulatedFully stimulatedFixation and permeabilization controls Flow Cytometry Basics Guide 8
Compensation controls – single stains todetermine fluorophore spectral overlap, have yougot the right stain?Positive and negative populationBright stainingSame fluorophoreCollect enough events Fc block – some cells have Fc receptors which canincrease background staining, including;MacrophagesB cellsNK cellsDendritic cells Fluorescence minus one control – aftercompensation, fluorescence spread can be aproblem. Set your gates accurately in a multicolorpanel using an FMO control, where all antibodiesbar one are included.Find out more atbio-rad-antibodies.com/fc-controlsFlow Cytometry Basics Guide 9
5. OptimizationGood sample prep – in order to measure singlecells and get reliable data, your sample also hasto be as good as it can be. Here are some tips tohealthy cells.Defrost cells quickly removing DMSOPrepare cell suspensions as gently as possible Use enzymes if necessary to extract cellpopulationsRemove contaminating tissueUse the appropriate anticoagulantAvoid vortexing and leaving a dry pelletKeep cells on ice if possible Include a viability dye – dead cells have increasedautofluorescence, bind antibodies non specifically,and reduce the dynamic range. Gating on forwardand side scatter may not be sufficient to remove thedead cells.Doublets – plot the height or width against the areato exclude doublets from your analysis to avoidunwanted false positives.Flow Cytometry Basics Guide 10
Collect enough cells – the number of cells youneed to collect during analysis to have significantdata can vastly differ depending on the sample andfrequency of your cells. The table below shows anexample of how the frequency of cells can affectthe number of cells collected. Knowing your cellfrequency will help your experiment.Starting PopulationFrequencyNumber ,0000.1%1,000Intracellular staining – extra steps such as fixationand permeabilization can alter your staining. Chooseyour fixative carefully as it can alter your staining andhow long you can store your sample.Permeabilization strength may need to be differentdepending on your antigen. Alcohol fixation andpermeabilization is best for DNA staining.Find out more atbio-rad-antibodies.com/flow-optimizationFlow Cytometry Basics Guide 11
6. Panel BuildingSimple rules to guide you through multi colorexperimental design.Fluorophores – separate fluorophore excitationacross lasers, and where possible, the emissionacross the detectors. This will minimize the amountof spillover.Antigen density – as a general rule it is best tomatch bright fluorophores (e.g. PE) with lowexpressing markers and dimmer fluorophores (e.g.Pacific Blue) with highly expressed markers.Expression patterns – place fluorophores withspillover on mutually exclusive markers andco-expressing markers should be identified usingfluorophores with little spillover. Unknown expressionand activation markers should also be placed awayfrom potential spillover conflicts.Dump channels – this removes all unwanted cellsby labeling them with the same fluorophore,allowing you to ignore both the cells and thefluorescence from those cells. A viability stain can beincluded in this channel for convenience.Flow Cytometry Basics Guide 12
Antibody titration – excess antibody can bind atlow affinity creating background fluorescence thatcan reduce the resolution of signal. Determine thebest antibody concentration by diluting the antibodyto the concentration that gives the best stain indexto both improve your experiment and save youmoney.Stain IndexFind out more atbio-rad-antibodies.com/flow-multicolorAntibody amount (µl)Online ResourcesCheck fluorophore excitation and emission spectracompatibility using our spectraviewer, or use ouronline panel builder to build panels in just a fewsimple antibodies.com/panel-builderFlow Cytometry Basics Guide 13
7. Simple Staining ProtocolsTo have the best staining, ensure samplepreparation is optimized before you start.These short protocols are a good starting point butsome optimization may be required depending onsample and antibody used.Surface staining1. Wash 1 x 106 cells and resuspend in cold PBS/BSA2. Incubate with antibody in a small volume for30 min at 4oC, avoiding direct sunlightFor indirect staining wash the cells in PBS/BSA, then add secondary reagent at vendorrecommended dilution3. Wash with cold PBS4. Fix if necessary in 1% PFA5. Acquire Flow Cytometry Basics Guide 14
Intracellular staining using Leucoperm1. Wash 1 x 106 cells and resuspend in PBS/BSA2. If required stain surface epitopes3. Wash cells in cold PBS/BSA4. Resuspend in Leucoperm Reagent A (fixative)5. Wash in PBS/BSA6. Resuspend in Leucoperm Reagent B(permeabilization reagent)7. Incubate with antibody in a small volume for30 min at 4oC, avoiding direct sunlight8. Wash with PBS9. AcquireOther fixatives and permeabilization reagents canbe used and some optimization may be requireddepending on the sample and location of epitope.Find out more atbio-rad-antibodies.com/fc-protocolsFlow Cytometry Basics Guide 15
Think Antibodies. Think Bio-Rad.Application guidesResourcesELISA, flow cytometry,IHC, western esearch areaantibodiesCancerImmunologyVeterinaryCell markerselectionguideMouse andHumanIn depthmini-reviewsNo experimentis completewithout all thepiecesTo request or download your copy of the flowcytometry guide go tobio-rad-antibodies.com/flowguideFlow Cytometry Basics Guide 16
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions. All trademarks used hereinare the property of their respective owner.bio-rad-antibodies.comBio-RadLaboratories, Inc.Bulletin 6977 Ver CUS/EG0719Sig 0119
your flow cytometry experiment: Unstained - determine where your cells are and what is negative. Isotype controls - determine specific surface staining, have you got the right isotype? Same species Same subclass Same fluorophore Biological controls - essential for most experiments, not just flow cytometry, make sure you