Transcription
Annex 1. TECHNICAL REQUIREMENTS (Based upon theinitial assessment)1
CONTENTS1.2.FUNCTIONAL REQUIREMENTS .31.1.Conceptual Architecture. 31.2.Users/Actors . 51.3.LIST OF REQUIREMENTS . 61.4.BUSINESS-PROCESSES . 29SOFTWARE RELATED NON-FUNCTIONAL REQUIREMENTS . 392.1.Requirements regarding system architecture . 392.2.Requirements regarding technology . 422.3.Requirements regarding interoperability . 452.4.Requirements regarding informational security . 452.5.Performance requirements . 473.Centralized Databases . 484.Software Backup . 495.Disaster Recovery and Business Continuity . 506.Software and User Documentation . 517.Testing, Quality Assurance and UAT . 528.Venue for Software Development/Customization. 559.License Agreement and Supply of Source Code . 5610. Software Support . 5711. System Lifetime . 5812. Scale up for The Entire Country . 5913. Requirements for Hardware. 6014. Server Room Requirements . 612
1. FUNCTIONAL REQUIREMENTS1.1. Conceptual ArchitectureFig.1 Conceptual architecture of VMISThe diagram showed in Fig. 1 represents the basic actors, which must be involved in the VMIS.The most important are warehouses at central (republican), regional, district levelsThe System will allow providing data on operations like receiving, transferring, write-off andinventory:-Through a graphical user interface – by entering needed data manually in a special electronicform. the system will perform some calculations automatically, based on entered data;At the primary level, in policlinics and vaccination cabinets the responsible users will have thepossibility to provide all needed data on vaccine consumption such as: data on patient, vaccine, dose,lot, series, etc. All consumption reporting may be done automatically by the System’s Analytic andReporting Module, as well as wastage calculations and other stock calculations, at any level.3
Fig. 2 Conceptual decomposition of the System’s levels (including all three implementationstages)Taking into account the As-Is situation in the Uzbekistan’s centers for Sanitary-EpidemiologicalSurveillance, it is recommended to implement the Vaccine Management Information System whichwould include at least the following major features: Tracking of the procured quantities of vaccines;Batch & Expiry Management;Management of the contracts related to the supply of vaccines;Stock management of vaccines at central and regional, district and local levels (receiving,transferring, write-off, inventory, consumption);Demand Forecasting and stock out (25% buffer) alarm system close to stock out;Search and reporting features for ensuring real-time access to the verification of availability ofvaccines at any level;Data analytics, Reporting and Dashboards.4
Fig. 3. Diagram of System components1.2. Users/ActorsError! Reference source not found.Fig. 4 below is a high-level representation of the users/actors ofthe VMIS. It depicts the external users, staff of the involved entities, support staff, and systemsinvolved in delivering the VMIS. The arrows (a generalization relationship) indicate organizationstructure to a certain extent, but are meant to represent the inheritance of access rights. The users5
presented will have their specific roles in the VMIS and each with its own functions andresponsibilities.The external information systems, which will be used to exchange data with, are not shown in theusers/actors diagram. This is because the data exchange between the VMIS and the external sourcesof information will be implemented through secured web-services.Fig. 4 Involved Actors1.3. LIST OF REQUIREMENTSThe VMIS shall be implemented as a modular platform based on Service Oriented Architecture (SOA)and that would allow the integration with other relevant ICT solutions in the health sector ofUzbekistan.VMIS as a modular platform must emphasize separating the functionality of the System intoindependent, interchangeable modules, such that each contains everything necessary to executeonly one aspect of the needed functionality.According to SOA principles, a service logically represents a business activity in real life with aspecified outcome. Thus, the System Architecture must allow exposing such services as VMIS mustallow in the future different user interfaces to work with the same data source.Also, VMIS must provide rich web interface which has many of the characteristics of desktop, butdelivered through a standard web browser by using extensive use of JavaScript, HTML5 and similartechnologies. In addition, the VMIS solution must be based on Responsive Web Design (RWD)approach, which allows web interfaces to render well on a variety of devices and windows or screensizes for mobile devices such as smartphones, tablets, etc.Each functional requirement is labelled with FRQ prefix and could have the following marks: (M) Mandatory “The System must .”;(HD) Highly Desirable “The System shall .”; and6
(D) Desirable “The System may .”;, where: MUST – means that the requirement is defined is an absolute have requirement;MUST NOT – means that the numbered requirement is defined is an absolute prohibition.SHOULD – means that there may exist valid reasons in particular circumstances to ignore aparticular item, but the full implications must be understood and carefully weighed beforechoosing a different course.Each functionality has the accompanying use-case diagram, which elaborates the requirement in amore detail way and presents the business context in which the System functionality is used. Thedetailed design that will be provided by the Contractor will have the detailed description of thefunctionalities.Functional Requirements for the Vaccine Supply Planning and Procurement ModuleIdentifierNameTypeDescriptionFRQ001Calendar of preventivevaccinationsMThe VMIS must provide a dedicated user interface forregistering the national Calendar of preventivevaccinations in Uzbekistan. Based on theaforementioned calendar, further, the calculation listof the needed vaccines is developed.FRQ002Data collection relatedto the vaccines demandMThe VMIS solution must provide a dedicated userinterface that allows users at certain level/medicalinstitution to specify the demand for vaccines.FRQ003Information on neededvaccinesMDuring the process of demand recording, the Systemwill register all information on requested vaccine,quantity, stock on-hand, and other relatedinformation to vaccines.Also the System will record the information relatedto the entity which requests the vaccines: ID andname of the institution, region, district, responsibleperson, etc.FRQ004Using official nationallist of vaccines inUzbekistanMThe System must operate only with the vaccines thatare officially registered in Uzbekistan.FRQ005Vaccine distribution listsMThe VMIS solution will allow responsible users tocreate and save distribution lists of vaccines.Republican level creates distribution lists for theregions.7
IdentifierNameTypeDescriptionRegional levels create distribution lists for districtlevels.District level creates distribution lists for itssubordinated medical institutions.FRQ006Data on budget forprocurement ofvaccinesMThe proposed System must allow MoH users to storeand manage information on approved budget for theprocurement of vaccines.FRQ007Previous ToRs related tothe procurement ofvaccinesMThe System must keep all previous ToRs related tothe procurement of essential vaccines during thepast periods.FRQ008Regulatory base relatedto the procurementprocess of vaccines inUzbekistanMThe System must keep all needed regulatorydocuments related to the procurement process. Theusers must have the possibility to access the legalinformation regarding the procurement process ofvaccines in Uzbekistan.FRQ009Management of ToRsMThe System must provide functionalities for creationand storage of ToRs for the procurement of vaccines.During this process, users must have access to thefollowing information managed by the System:-Approved consolidated report on vaccinesdemand;- Indicative prices;- Approved budget;- Required quantities.Similar to the formation and approval of thenomenclature, the System must be integrated with anintranet that would allow publishing the ToRs as partof knowledge management and experience sharingbetween regions (internal process only )FRQ010Publish ToRsDFRQ011Approval of the ToRsDThe relevant user from MoH must have the possibilityto approve the ToRs.FRQ012Record procurementrequests tointernationalorganizationsDThe System must allow MoH users to document therequests for procurement to the internationalorganizations. For this purpose, the System will recordinto the database all the data related to a request andwill allow to upload and save all needed documents.FRQ013Management ofestimatesMAll estimates should be submitted through electronicdocument management workflows.FRQ014Contract managementDThe System will provide functionalities formanagement of contracts with Suppliers of vaccines.It will allow users to upload scanned copies of8
IdentifierNameTypeDescriptioncontracts and filling all related metadata, as well assetting certain alert rules (e.g. alerts related to thescheduled deliveries of vaccines).FRQ015Tracking relations withsuppliersMThe System will provide all necessary features andreports for tracking relations with the Suppliers.Fig. 6 Use Case diagram related to the Procurement and Supply Manager’s main activities9
Functional Requirements for the Module for Collecting Data on Needs for ing dataregarding the needs forvaccines in the medicalinstitutionsMThe System must provide a dedicated module andgraphical user interface for the data collectionprocess regarding the needs for vaccines in medicalinstitutions.FRQ017Collecting data fromregions/medicalinstitutionsMThe users from the regions/medical institutions musthave the possibility provide information on thevaccines, required quantity and related plannedindicators. The System will automatically calculate allthe necessary amounts of vaccines.FRQ018Taking into account allrelevant informationMThe VMIS software solution must take into accountthe stock on-hand of vaccines and their shelf life,planned budget for procurement, planned suppliesand other relevant parameters (to be establishedduring the analysis and design phase).FRQ019Confirmation by usingdigital signatureMAll provided data regarding the needs for vaccines inthe medical institutions/regions will be confirmed bythe user by applying the digital signature.FRQ020Data consolidationMThe System will automatically consolidate allcollected data from the regions.Therefore VMIS will consolidate all saved requests ofvaccines from all the regions and will apply allnecessary calculation algorithms in order to create aconsolidate list of vaccines needed to be procured.FRQ021Consolidated Report onvaccine demandsMAll collected data regarding the needs for vaccines inthe regions will be consolidated in a form of Report,which can be adjusted until it is digitally signed.FRQ022Approval by using digitalsignatureMThe approval process will be carried out through thedigital signatures of the responsible users.In this regard, the VMIS will use the digital signature,which is issued by the Scientific Information Centerof New Technologies of the State Tax Committee ofthe Republic of Uzbekistan and its regional offices.FRQ023Comparative analysisand other relevantanalyticsMThe System must provide reporting functionalitiesand Business Intelligence tools in order to be possibleto make comparative analysis of the informationregarding the needs for vaccines vs information ofthe past periods.FRQ024Additional or refinedrequestsMThe system should provide the possibility to make anadditional or refined request regarding the needs for10
IdentifierNameTypeDescriptionvaccines, depending on possible savings or deficitsduring the procurement process.Fig. 7 Use case diagram related to the process of requesting vaccinesFunctional Requirements for the Warehousing and Inventory ModuleThis Module represents in fact the stock management of vaccines and other vaccines, also known asinventory management, and involves all the policies, procedures, and techniques used to maintain the11
optimum amount of each item in stock. It involves ordering, receiving, storing, issuing, and reorderingitems. Stock and inventory management are the core of the vaccines supply system.uc WarehousingSystemConfirm receiv ing ofv accinesWarehouseAdministratorSet minimal andmaximal stock limitper each medicalproduct«include»(fromActors)Shipping use Operator(fromActors)Write-Off operation«include»Warehouse ManagerUser(fromActors)«include»Stock Inv entory«include»«include»Chat w ith otherusersRequest UserAccountconfigurationRun Reportse.g. In cases when a new Warehouse Operator isemployed, the Warehouse Manager will make arequest to the System Administrator in order tocreate a new User Account and to configure allnecessary access rights.Also, in case an already registered WarehouseOperator leaves, the Warehouse Manager mustnotify the System Administrator in order todeactivate the account.Fig. 8 Use case diagram related to the Warehousing and Inventory ModuleIdentifierNameTypeDescriptionFRQ025Real-time informationMThe module should reflect in real time the receipt ofvaccines, real balances, and shipping requests.FRQ026Warehousing operationsMThe System must offer all necessary functionalitiesrelated to the stock management of vaccines:receiving, shipping, write-off and inventoryoperations. In other words, the proposed softwaresolution must assure the management of stocktransactions: entries into and exits from stock,transfers between warehouses, stock inventory and12
IdentifierNameTypeDescriptionthe possibility to automatically generate thenecessary corrections by each stock item as aconsequence of inventory differences.FRQ027Losses and adjustmentsoperationsMLosses and adjustments operations is related to anyissue out of the store for reasons other thanconsumption within the warehouse, e.g.:-FRQ028Analytical informationMFRQ029justifying documentsrelated to the transactionsMChanges in the stock due to removal of expireditems, damaged items, theft, or adjustments tostock numbers during stock-taking;- Redistribution to other warehouses/medicalinstitutions are also recorded as a loss;- Gains in stock quantities by means other thannormal procurement channels;In case of stock entries, the proposed VMIS softwaresolution must allow the visualization of theagreement/contract based on which the vaccineswere delivered. The system must allow the analysisof the agreement – what was contracted, how muchwas delivered and how much must be delivered atthe level of each vaccine from the agreement, whatpayments must be performed (in advance, at themoment of service providing, final payment, etc). AContractor can have several agreements, and eachagreement can have several addenda.The System will automatically generate justifyingdocuments for the managed transactions.For each (transaction) movement of vaccines, theSystem must assure the automatic generation ofaccounting documents. .FRQ030Warehouses’ManagementMThe System must allow the registering of unlimitednumber of warehouses.The System must allow users to define thewarehouses with which they currently work (e.g.central, regional or local level).FRQ031Configuration ofwarehouses’ locationsMThe proposed VMIS software solution must allowusers to define the locations in which they will storethe items in the warehouse.FRQ032Shelf-life monitoringMThe System must provide functionalities that wouldallow the Shelf-life monitoring and alerting.FRQ033Dashboard: Alerting andReportingMThe System’s dashboard must highlight thepercentage of vaccines that are in risk of expiry.13
IdentifierNameTypeDescriptionSystem’s Reports must alert the user that particularproducts will be available for at least 3 months basedon Average Monthly Consumptions.This must prompt the managers to seeking ways totransfer the product to/from another medicalinstitution.FRQ034Minimum and Maximumstock levelsMThe System must alert the user when stock reaches aminimum or maximum level and assist in developingthe request to higher level for vaccinesreplenishment.FRQ035Interface for the PublicAccessMThe System must provide a dedicated interface forpublic access to the information about theavailability of vaccines in certain warehouse.FRQ036Tracking. Traceability.MThe System must provide functionalities that wouldallow tracking of vaccines according to variouscriteria such as Lot/Serial Number. Therefore, theSystem must ensure the traceability of the entirechain of vaccines.FRQ037Re-distribution operationsof vaccinesMThe System must allow re-distribution operations ofthe vaccines. (e.g. re-distribution of vaccines fromone medical institution to another).FRQ038Confirmation of receivingvaccines in the warehouseMThe System will provide confirmation functionalitiesfor the warehouses’ users which are receivingvaccines. Thus, the user will have to confirm in theSystem whether all the received quantities andquality of goods are according to the distributiondocument.FRQ039Unique nomenclature ofvaccinesMThe System must keep a unique officialnomenclature of vaccines used in Uzbekistan.FRQ040Traceability Stock CardsMThe System must provide functionalities formanaging the so-called Stock Cards. Stock cards trackthe movement of vaccines in the warehouses andone card must be kept for each item in thewarehouse.Each time a transaction takes place, it must beavailable to be visualized on the stock card.FRQ041Stock Card StructureMThe tentative description of the Stock Card ispresented below and will be finally defined duringthe Analysis and Design Phase of the Project:14Header information about the warehouse;Item description;
IdentifierNameTypeDescription- Pack size;- Code number;- Special storage conditions;- Unit of issue;- Average Monthly Consumption;- Minimum stock level;- Date;- Locations From/To;- Quantity in;- Quantity out;- Losses/adjustments;- Expiry date;- Batch or lot number;- Remarks;The proposed software solution must assure the fastvisualization of basic information associated to anitem (vaccine product).FRQ042Fast visualization of thestock itemMFRQ043Grouping of vaccinesMThe System must allow the classification of vaccinesinto groups of products and the definition of groupsof products.FRQ044Standard Reports onstocksMThe System will include standard reports regardingstocks and/or movements of vaccines, allowing tospecify the period of time.FRQ045Search featureMThe proposed VMIS software solution must contain amulticriteria search engine and offer users thepossibility of selection or editing of information bycertain criteria.FRQ046Update information onstocksMThe System must provide a dedicated functionalityfor the medical institutions and warehouses thatwould allow users to update information on stockon-hand of vaccines and vaccines.FRQ047Submit requests forvaccines deliveryMThe proposed software solution must providefunctionalities for medical institutions to submitrequests for vaccines delivery, including all relateddocuments.FRQ048Single window for vaccinesorderingMThe system should be user-oriented and serve as thesingle window for ordering vaccines. It should reflectthe stock on-hand, ordered vaccines and estimatedarrival time of the goods in the medical institution, aswell as statistics on the average monthlyconsumption.15
IdentifierNameTypeDescriptionFRQ049Integration with HMISMThe System must provide a dedicated API forelectronic data exchange with HMIS.FRQ050Redistribution of vaccinesMThe system may propose how to redistributevaccines, based on data regarding the needs forvaccines in other medical institutions, consumptionand stock on-hand.Functional Requirements for the Administration ModuleIdentifierNameTypeDescriptionFRQ051Manage DocumentManagementMThe administrator shall be able to define a newdocument template that is created or uploaded in theSystem using the administrative/managementconsole of the System. The VMIS shall allowconfiguration of different data for differentdocuments, such as field types, validation rules,ranges, possible values, instructions and errormessages.FRQ052Manage alerts/internalnotificationsMAccording to the internal processes, different usersmay need to be informed of various tasks awaitingtheir action in the System e.g. received vaccines in awarehouse that are awaiting confirmation from theside of Operator. For this purpose the System willallow the administrator to manage the configurationof alerts using the administrative/managementconsole.FRQ053Manage organizations(entities) and unitsMThe System shall enable managing information aboutrelevant organizations and organizational units. Forinstance, the relevant authorities shall have their ownprofiles that they use to log into the System andupdate only their own information (Email Contact,Telephone Contact, Website Contact, PhysicalLocation, Organizational Units, Branches).FRQ054Content managementMThe System shall allow administrator to manage staticcontent without having to change the software code.FRQ055Manage users andauthorizationsMThe management of user’s roles and permissions interms of accessing the functionalities and data savedin the System – manage user rights through theroles/groups, will be available using theadministrative/management console.16
IdentifierFRQ056NameLogs MonitoringTypeDescriptionMThe Administrator will be able to monitor all actionsthat occur in the System, including those with themost important events with the legal impact. For thispurpose, the System will have its own loggingmechanism.Fig. 10. Use Case Diagram related to the Document management17
Fig. 11. Use Case Diagram related to the Management of alertsReferring to the above use case diagram (FigFig), IT administrator must have possibility to managedifferent types of alerts in the System. For example, the administrator shall be able to manage alerts thatare related to the change of status of a document or the fact of receiving vaccines in the warehouse – anoperation that is awaiting confirmation.18
Fig. 12. Use Case Diagram “Manage Entities (Organizations)”19
Fig. 13. Use Case Diagram “ Manage users and list of vaccines”Users will be managed in a centralized manner. the VMIS solution must offer a possibility for managinguser accounts, user roles (e.g. to create, update, change status and delete) and security policy, by meansof defining the permissions for user roles and users to access the functionalities and data saved in theSystem. The users shall be able to authenticate through login and password.20
Functional Requirements for the Reporting ModuleIdentifierFRQ057NameCommon reportfunctionalitiesTypeDescriptionMThe System will allow defining parameters forgenerating reports, for instance supplies per vaccinetype, time period, public authority, classifier, etc.The VMIS will enable printing reports and exportingreports using HTML, Excel, Word, xml and pdf).Also, the System will provide support for subscribingto the reports, by means of receiving generatedreports by email in regular intervals, prepared basedon defined criteria in the subscription.FRQ058Analytical reportsMThe System must be capable of producing reportsdesegregated by type of source of supply. A report pereach warehouse that groups the quantity of vaccinesreceived from different sources (procured,transferred in from other facilities, received from thecentral warehouse, etc.) should be produced.FRQ059List reportsMThe VMIS will allow generating list reports (e.g. List oftransactions, List of delivered vaccines, List ofcancelled transactions, etc.). The Contractor willprovide up to 10 reports to list information from theSystem.FRQ060Statistical reportsMThe Contractor will implement up to 15 differentstatistical reports (standard and advanced) that will bedefined as part of the detailed design process.FRQ061Time series reportsMThe System will enable generating reports with timeseries of key performance indicators and graphicrepresentations. The Contractor will develop up to 7time-series reports.FRQ062Administrative reportsMThe System will enable generating administrativereports on completed workflow statuses by users,based on user activity, completed transactions byusers and other standard administrative reports.The use-case diagrams related to the reporting module that are included below provide some examplesof the reports that are expected to be provided by the Contractor. The intention is that Contractorunderstands the complexity level to be able to estimate efforts for the requested number of reports. Thefinal list and design of the reports will be determined during the detailed design of the System.21
Fig. 12 Use Case Diagram related to the Common Reporting FunctionalitiesThe System will support drill-down option in the report, from some general level to the detailedrepresentation level. For example, to prepare a report on level of business activity group and see thenumber of transactions related to the delivery of vaccines in certain time period, and then to drill downover categories to the vaccines type.The System will enable searching generated reports and will implement web-based reporting interface.The report templates will be managed by the administrator and the System must include a tool (web userinterface) for designing reports.The VMIS System will support normal or cross-tab reports. User must have possibility to reduce processingimpact during core business hours by scheduling large reports to generate during off-peak hours.22
Fig. 23. Use Case Diagram related to Analytical ReportsFig. 346. Use Case Diagram related to List Reports23
Fig. 1745. Use Case Diagram related to the Statistical ReportsFig. 18.Use Case Diagram related to the Time Series Reports24
Fig. 19. Use Case Diagram related to the Administrative al support fordecision-makersMThe proposed VMIS solution must make available tousers capacities of managerial analysis supported bycubes of pre-processed data.FRQ064Cubes of specialized databy each area with thepossibility ofcustomizationMThe proposed solution must contain cubes ofspecialized data by each area with the possibility ofcustomization of views of analysis (table or graphic).FRQ065Filtering and sortingMThe proposed solution must allow users to establishfiltering and sorting criteria at the moment ofrunning reports.Also, the System must offer a reporting tool that willallow users do design customized reports and to savetemplates. Also there must be possible to generatead-hoc reports.FRQ066Synthetic reports withaggregated dataMThe proposed VMIS solution must allow theextraction of synthetic reports with aggregated datafrom various activities and modules.25
FRQ067Schemes of fast definitionMThe System must allow the use of schemes of fastdefinition of requirements of each specific data field(field covered by the system).FRQ068Extraction of reports inreal timeMThe System must allow the extraction of reports inreal time by activities designed for the MoHmanagement.FRQ069Adding new types ofreportsMThe proposed solution must assure the possibility ofadding new types of reports in a simple and intuitivemanner.FRQ070Customized reportingMThe proposed solution must allow the extraction ofany types of reports depending on requirements.FRQ071Exporting reports in otherformatsMThe reports must be exportable in PDF, MS Excel, andautomated delivery mechanisms (e.g. e-mail).FRQ072Definition of templatesMThe solution must allow definition of templates
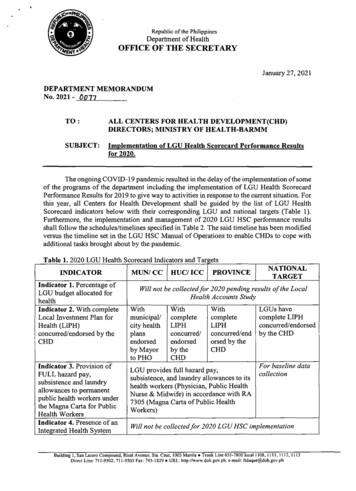
M The VMIS software solution must take into account the stock on-hand of vaccines and their shelf life, planned budget for procurement, planned supplies and other relevant parameters (to be established during the analysis and design phase). FRQ019 Confirmation by using digital signature M All provided data regarding the needs for vaccines in