Transcription
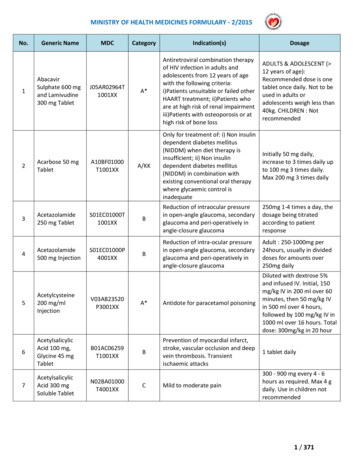
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.1234Generic NameAbacavirSulphate 600 mgand Lamivudine300 mg TabletAcarbose 50 mgTabletAcetazolamide250 mg TabletAcetazolamide500 mg C01000T1001XXS01EC01000P4001XX5Acetylcysteine200 d 100 mg,Glycine 45 mgTabletB01AC06259T1001XX7AcetylsalicylicAcid 300 mgSoluble A*Antiretroviral combination therapyof HIV infection in adults andadolescents from 12 years of agewith the following criteria:i)Patients unsuitable or failed otherHAART treatment; ii)Patients whoare at high risk of renal impairmentiii)Patients with osteoporosis or athigh risk of bone lossADULTS & ADOLESCENT ( 12 years of age):Recommended dose is onetablet once daily. Not to beused in adults oradolescents weigh less than40kg. CHILDREN : NotrecommendedA/KKOnly for treatment of: i) Non insulindependent diabetes mellitus(NIDDM) when diet therapy isinsufficient; ii) Non insulindependent diabetes mellitus(NIDDM) in combination withexisting conventional oral therapywhere glycaemic control isinadequateInitially 50 mg daily,increase to 3 times daily upto 100 mg 3 times daily.Max 200 mg 3 times dailyBReduction of intraocular pressurein open-angle glaucoma, secondaryglaucoma and peri-operatively inangle-closure glaucoma250mg 1-4 times a day, thedosage being titratedaccording to patientresponseBReduction of intra-ocular pressurein open-angle glaucoma, secondaryglaucoma and peri-operatively inangle-closure glaucomaAdult : 250-1000mg per24hours, usually in divideddoses for amounts over250mg dailyA*Antidote for paracetamol poisoningDiluted with dextrose 5%and infused IV. Initial, 150mg/kg IV in 200 ml over 60minutes, then 50 mg/kg IVin 500 ml over 4 hours,followed by 100 mg/kg IV in1000 ml over 16 hours. Totaldose: 300mg/kg in 20 hourBPrevention of myocardial infarct,stroke, vascular occlusion and deepvein thrombosis. Transientischaemic attacks1 tablet dailyMild to moderate pain300 - 900 mg every 4 - 6hours as required. Max 4 gdaily. Use in children notrecommendedC1 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.8Generic NameAcitretin 10 A*i) Severe form of psoriasis includingerythrodermic psoriasis and localor generalized pustular psoriasis.ii) Severe disorders ofkeratinization, such as -congenitalichthyosis -pityriasis rubra pilaris Darier's disease -other disorders ofkeratinization which may beresistant to other therapies9Acitretin 25 mgCapsuleD05BB02000C1002XXA*i) Severe form of psoriasis includingerythrodermic psoriasis and localor generalized pustular psoriasis.ii) Severe disorders ofkeratinization, such as -congenitalichthyosis -pityriasis rubra pilaris Darier's disease -other disorders ofkeratinization which may beresistant to other therapies10Acriflavine 0.1%LotionD08AA03000L6001XXC Infected skin, lesions, cuts,abrasions, wounds and burns.11Actinomycin D(Dactinomycin)500 mcg/mlInjectionL01DA01110P4001XXAi) For solid tumours ii) Gestationaltrophoblastic diseaseDosageADULT: initially 25-30 mgdaily for 2-4 weeks, thenadjusted according toresponse, usually withinrange 25-50 mg daily forfurther 6-8 weeks (max: 75mg daily). In disorders ofkeratinization, maintenancetherapy of less than20mg/day and should notexceed 50mg/day CHILD:0.5mg/kg daily occasionallyup to 1 mg/kg daily to amax. 35 mg daily for limitedperiodsADULT: initially 25-30 mgdaily for 2-4 weeks, thenadjusted according toresponse, usually withinrange 25-50 mg daily forfurther 6-8 weeks (max: 75mg daily). In disorders ofkeratinization, maintenancetherapy of less than20mg/day and should notexceed 50mg/day CHILD:0.5mg/kg daily occasionallyup to 1 mg/kg daily to amax. 35 mg daily for limitedperiodsApply undiluted three timesdaily to the affected part.i) ADULT: 500 mcg IV dailyfor max of 5 days. CHILD: 1.5mg/m2 once every 3 weeks(if weight less than 10 kg, 50mcg/kg) ii) 500 mcg IV onDays 2, 4, 6, 8, 10, repeatevery 7 - 10 days or 500 mcgIV bolus on Days 1 and 2,repeat every 15 days2 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.1213Generic NameAcyclovir 200 mgTabletAcyclovir 200mg/5 ategoryIndication(s)A/KKi) Mucocutaneous Herpes Simplexinfection in immunocompromisedand AIDS patients ii) Primary andrecurrent Varicella Zoster infectionin immunocompromised and AIDSpatients iii) Severe Kaposi VaricellaEruption (Eczema herpeticum) iv)Severe primary HSV infections (eg.Neonatal herpes, encephalitis,eczema herpeticum, genital herpes,gingival stomatitis, vaginal deliverywith maternal vulva herpes) v)Severe and complicated varicellainfection (eg. Encephalitis, purpurafulminans) vi) Severe zosterinfection in paediatrics (eg.Encephalitis, purpura fulminans,immunocompromised patients andfacial, sacral and motor zoster)A*i) Mucocutaneous Herpes Simplexinfection in immunocompromisedand AIDS patients ii) Primary andrecurrent Varicella Zoster infectionin immunocompromised and AIDSpatients iii) Severe Kaposi VaricellaEruption (Eczema herpeticum) iv)Severe primary HSV infections (eg.Neonatal herpes, encephalitis,eczema herpeticum, genital herpes,gingival stomatitis, vaginal deliverywith maternal vulva herpes) v)Severe and complicated varicellainfection (eg. Encephalitis, purpurafulminans) vi) Severe zosterinfection in paediatrics(eg.Encephalitis, purpurafulminans, immunocompromisedpatients and facial, sacral andmotor zoster)Dosagei) ADULT: initially 400 mg 5times daily for 7 - 14 days.CHILD less than 2 years: 200mg 4 times daily, CHILDmore than 2 years: 400 mg 4times daily ii), iii) and iv)ADULT: 200 - 400 mg 4times daily. CHILD: less than2 years, half adult dose;more than 2 years, adultdose v) ADULT: 800 mg 5times daily for 7 days vi)ADULT: 20 mg/kg(maximum: 800 mg) fourtimes daily for 5 days, CHILD6 years: 800 mg four timesdaily. CHILD less than 2years; 400mg 4 times daily,more than 2 years; 800mg 4times dailyi) ADULT: initially 400 mg 5times daily for 7 - 14 days.CHILD less than 2 years: 200mg 4 times daily, CHILDmore than 2 years: 400 mg 4times daily ii), iii) and iv)ADULT: 200 - 400 mg 4times daily. CHILD: less than2 years, half adult dose;more than 2 years, adultdose. v) ADULT: 800 mg 5times daily for 7 days vi)ADULT: 20 mg/kg(maximum: 800 mg) fourtimes daily for 5 days, CHILD6 years: 800 mg four timesdaily. CHILD: less than 2years; 400mg 4 times daily,more than 2 years; 800 mg 4times daily3 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)14Acyclovir 250 mgInjectionJ05AB01000P4001XXA*Treatment and prophylaxis ofherpes simplex inimmunocompromised, severeinitial genital herpes and Varicella Zoster15Acyclovir 3% EyeOintmentS01AD03000G5101XXA*Only for the treatment of herpessimplex keratitisA*Herpes simplex infections of theskin, including initial and recurrentlabial and genital herpes simplexinfections16Acyclovir 5%CreamD06BB03000G1001XXDosageADULT: 5 mg/kg by IVinfusion 8 hourly for 5 days,doubled to 10mg/kg every 8hourly in varicella-zoster inthe immunocompromisedand in simplex encephalitis(usually given for at least 10days in encephalitis;possibly for 14 - 21 days).NEONATE & INFANT up to 3months with disseminatedherpes simplex: 20mg/kgevery 8 hourly for 14 days(21 days in CNSinvolvement), varicellazoster 10-20mg/kg every 8hourly usually for 7 days.CHILD, 3 months - 12 years:Herpes simplex or VaricellaZoster: 250 mg/m2 8 hourlyfor 5 days, doubled to 500mg/m2 8 hourly forvaricella-zoster in theimmunocompromised andin simplex encephalitis(usually given for 10 days inencephalitis)Apply 1 cm 5 times daily.Continue for at least 3 daysafter healingApply every 4 hours for 5 10 days4 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.17Generic NameAcyclovir 800 osageA/KKi) Mucocutaneous Herpes Simplexinfection in immunocompromisedand AIDS patients ii) Primary andrecurrent Varicella Zoster infectionin immunocompromised and AIDSpatients iii) Severe Kaposi VaricellaEruption (Eczema herpeticum) iv)Severe primary HSV infections (eg.Neonatal herpes, encephalitis,eczema herpeticum, genital herpes,gingival stomatitis, vaginal deliverywith maternal vulva herpes) v)Severe and complicated varicellainfection (eg. Encephalitis, purpurafulminans) vi) Severe zosterinfection in paediatrics (eg.Encephalitis, purpura fulminans,immunocompromised patients andfacial, sacral and motor zoster)i) ADULT: initially 400 mg 5times daily for 7 - 14 days.CHILD less than 2 years: 200mg 4 times daily, CHILDmore than 2 years: 400 mg 4times daily ii), iii) and iv)ADULT: 200 - 400 mg 4times daily. CHILD: less than2 years, half adult dose;more than 2 years, adultdose v) ADULT: 800 mg 5times daily for 7 days vi)ADULT: 20 mg/kg(maximum: 800 mg) fourtimes daily for 5 days, CHILD6 years: 800 mg four timesdaily. CHILD less than 2years; 400mg 4 times daily,more than 2 years; 800mg 4times daily5 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategory18Adalimumab 40mg InjectionL04AB04000P5001XXA*19Adapalene 0.1%CreamD10AD03000G1001XXA*20Adapalene 0.1%GelD10AD03000G3001XXA*Indication(s)i) Third line treatment of: - Severerheumatoid arthritis - Psoriaticarthritis - Ankylosing spondylitisafter failure of conventionalDMARDs or other biologics. ii)Treatment of adults with moderateto severe chronic plaque psoriasiswho have not responded to, havecontraindication or are unable totolerate phototherapy and/orsystemic therapies includingacitretin, methotreaxate andcyclosporine. iii) Crohn's Disease a) For treatment of moderately toseverely active Crohn’s Disease inadult patients who haveinadequate response toconventional therapy;b) For treatment of moderately toseverely active Crohn’s Disease inadult patients who have lostresponse to or are intolerant toinfliximab. iv) Ulcerative Colitis For treatment of moderately toseverely active ulcerative colitis inadult patients who have had aninadequate response toconventional therapy includingcorticosteroids and 6mercaptopurine or azathioprine, orwho are intolerant to or havemedical contraindications for suchtherapiesAcne vulgaris where comedones,papules and pustules predominatein those sensitive to benzoylperoxide or topical tretinoin [thirdline treatment]Acne vulgaris where comedones,papules and pustules predominatein those sensitive to benzoylperoxide or topical tretinoin [thirdline treatment]Dosagei) Severe rheumatoidarthritis, Psoriatic arthritis,Ankylosing spondylitis :Subcutaneous 40 mg everyother week ii)Chronicplaque psoriasis : Initial, 80mg SC, followed by 40 mg SCevery other week startingone week after the initialdose. iii) & iv) Crohn’sdisease & Ulcerative colitis:160mg at week 0 (dose canbe administered as fourinjections in one day or astwo injections per day fortwo consecutive days) and80mg at week 2. Afterinduction treatment, therecommended maintenancedose is 40mg every otherweek via subcutaneousinjection.Apply once daily to theaffected areas after washingat bedtimeApply once daily to theaffected areas after washingat bedtime6 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.21Generic NameAdefovir Dipivoxil10 mg ) Treatment of chronic HBeAgpositive and HBeAg negativehepatitis B infection in adults withcompensated liver function(lamivudine should be tried first) ii)Lamivudine-resistant chronichepatitis B virus infection witheither compensated ordecompensated hepatitis function(only by hepatologist andgastroenterologist for approvedindications)22Adenosine3mg/ml InjectionC01EB10000P3001XXBRapid conversion of paroxysmalsupraventricular tachycardia tosinus rhythm23Adrenaline Acid(Epinephrine)Tartrate 1 mg/mlInjectionC01CA24123P3001XXBCardiopulmonary resuscitation24Agomelatine 25mg TabletN06AX22000T1001XXA*Major depression25Albendazole 200mg TabletP02CA03000T1001XXC i) Single or mixed infestations ofintestinal parasites ii) StrongyloidesinfectionDosageAdult (18-65 years): 10mgOnce Daily Renal DoseAdjustment : 10mg every48hours (30-49ml/min);10mg every 72hours (1029ml/min); 10mg every 7days (Hemodialysis)ADULT: Initially: 3 mg givenas a rapid IV bolus (over 2seconds). Second dose: Ifthe first dose does not resultin elimination of thesupraventricular tachycardiawith in 1 or 2 minutes, 6 mgshould be given also as arapid IV bolus. Third dose: Ifthe second dose does notresult in elimination of thesupraventicular tachycardiawithin 1-2 minutes, 12 mgshould be given also as arapid IV bolus1 mg by intravenousinjection repeated every 3-5minutes according toresponseThe recommended dose is25mg once daily at bedtime,maybe increased to 50mgonce daily at bedtime.i)Child 12-24months: 200mgas a single dose ii) Adult &Child above 2 years: 400mgas a single dose for 3consecutive days; Child 12 24months: 200mg as asingle dose for 3consecutive days7 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)26Albendazole 200mg/5 mlSuspensionP02CA03000L8001XXC i) Single or mixed infestations ofintestinal parasites ii) Strongyloidesinfection27Alcohol 70%SolutionD08AX08000L9901XXC Use as antiseptic and disinfectantA*Osteoporosis in post menopausalwomen with a history of vertebralfracture and whom oestrogenreplacement therapy iscontraindicated. Review treatmentafter 2 years and if there is positiveresponse, treatment may becontinued up to 5 years and thenre-evaluate. Treatment should bestopped if there is no positiveresponse after 5 years. Otherwise,patient needs to be given drugholiday for 1 to 2 years and thencontinue treatment shall thebenefit outweigh the risk.A*Osteoporosis in post menopausalwomen with a history of vertebralfracture and whom oestrogenreplacement therapy iscontraindicated. Review treatmentafter 2 years and if there is positiveresponse, treatment may becontinued up to 5 years and thenre-evaluate. Treatment should bestopped if there is no positiveresponse after 5 years. Otherwise,patient needs to be given drugholiday for 1 to 2 years and thencontinue treatment shall thebenefit outweigh the risk.2829AlendronateSodium 70 mgandCholecalciferol5600 IU TabletAlendronateSodium 70 Child 12-24months: 200mgas a single dose ii) Adult &Child above 2 years: 400mgas a single dose for 3consecutive days; Child 12 24months: 200mg as asingle dose for 3consecutive daysApply to the skin undilutedor when needed1 tablet once weekly[70mg/5600 IU]. Patientshould receive supplementalcalcium or vitamin D, ifdietary vitamin Dinadequate. The tabletshould be taken at least halfan hour before the firstfood, beverage, ormedication of the day withplain water only. Tofacilitate delivery tostomach and thus reducethe potential for esophagealirritation, it should only beswallowed upon arising forthe day with a full glass ofwater and patient shouldnot lie down for at least 30minutes and until after theirfirst food of the day.70 mg once weekly. Swallowthe tablet whole with a fullglass of plain water only onan empty stomach at least30 minutes before breakfast(and any other oralmedication); stand or situpright for at least 30minutes and do not lie downuntil after eating breakfast8 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.30Generic NameAlfacalcidol 0.25mcg dol 1mcg CapsuleA11CC03000C1002XXA/KK32Alfacalcidol 2mcg/ml DropsA11CC03000D5001XXA*33Alfacalcidol 2mcg/ml InjectionA11CC03000P3001XX34Alfentanil HCl 0.5mg/ml InjectionN01AH02110P3001XX35Alfuzosin HCl 10mg TabletG04CA01110T1001XXA*Indication(s)i) Renal osteodystrophy in patientson haemodialysisii) Hypoparathyroidism andpseudohypoparathyroidismiii) Adjunct to the management oftertiary hyperparathyroidismiv) Rickets and osteomalaciav) Osteoporosisi) Renal osteodystrophy in patientson haemodialysisii) Hypoparathyroidism andpseudohypoparathyroidismiii) Adjunct to the management oftertiary hyperparathyroidismiv) Rickets and osteomalaciav) Osteoporosisi) Renal osteodystrophy in patientson haemodialysisii) Hypoparathyroidism andpseudohypoparathyroidismiii) Adjunct to the management oftertiary hyperparathyroidismiv) Rickets and osteomalaciav) OsteoporosisTreatment of: i) Renalosteodystrophy in patients onhaemodialysisii) Hypoparathyroidism andpseudohypoparathyroidismiii) Adjunct to the management oftertiary hyperparathyroidismiv) Rickets and osteomalaciav) OsteoporosisDosageInitial dose ADULT andCHILD above 20kg bodyweight: 1 mcg daily; CHILDunder 20kg body weight:0.05mcg/kg/day.Maintenance dose : 0.25mcg to 2 mcg dailyInitial dose ADULT andCHILD above 20kg bodyweight: 1 mcg daily; CHILDunder 20kg body weight:0.05mcg/kg/day.Maintenance dose : 0.25mcg to 2 mcg dailyNEONATES : 0.1 mcg/kg/dayAdult: Initially, 1 mcg daily.Maintenance: 0.25-1 mcgdaily. Child: Prematureinfants and neonates: 0.050.1 mcg/kg daily; 20 kg:0.05 mcg/kg daily. Elderly:0.5 mcg daily.A*For use as short acting narcoticanalgesic in short procedures andday-care surgical proceduresInitial dose: 20 - 40 mcg/kg.Supplemental dose: 15mcg/kg or infusion 0.5 - 1.0mcg/kg/minA*Treatment of functional symptomsrelated with benign prostatichypertrophy (BPH)10 mg once a day pre bed9 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)36Alglucosidasealfa 5 mg/mlInjectionA16AB07000P4001XXA*Infantile-onset Pompe disease37Alkaline NasalDoucheR01A000999L5001XXBTo remove nasal plug38Allopurinol 100mg TabletM04AA01000T1002XX39Allopurinol 300mg TabletM04AA01000T1001XXA/KKA/KKi) Frequent and disabling attacks ofgouty arthritis (3 or moreattacks/year). ii) Clinical orradiographic signs of erosive goutyarthritis. iii) The presence oftophaceous deposits. iii) Uratenephropathy. iv) Uratenephrolithiasis. v) Impendingcytotoxic chemotherapy orradiotherapy for lymphoma orleukaemiai) Frequent and disabling attacks ofgouty arthritis (3 or moreattacks/year). ii) Clinical orradiographic signs of erosive goutyarthritis. iii) The presence oftophaceous deposits. iii) Uratenephropathy. iv) Uratenephrolithiasis. v) Impendingcytotoxic chemotherapy orradiotherapy for lymphoma orleukaemiaDosage20 mg/kg of body weightadministered once every 2weeks as an intravenousinfusion. Monitoring It issuggested that patients bemonitored periodically forIgG antibody formation.Patients who experienceInfusion-associatedreactions suggestive ofhypersensitivity may betested for IgE antibodies toalglucosidase alfa. Treatedpatients who experience adecrease in benefit despitecontinued treatment withAlglucosidase Alfa, in whomantibodies are suspected toplay a role, may be testedfor neutralization of enzymeuptake or activity.To be diluted with an equalvolume of warm waterbefore useInitial dose: 100-300 mgdaily. Maintenance: 300-600mg daily. Maximum: 900 mgdailyInitial dose: 100-300 mgdaily. Maintenance: 300-600mg daily. Maximum: 900 mgdaily10 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)40All-Trans RetinoicAcid 10 mgCapsuleL01XX14000C1001XXA*41Alprazolam 0.25mg TabletN05BA12000T1001XXA/KKAnxiety disorders42Alprazolam 0.5mg TabletN05BA12000T1002XXAAnxiety disorders43Alprazolam 1 mgTabletN05BA12000T1003XXAAnxiety disorders44Alprostadil 500mcg/ml InjectionC01EA01000P3001XXA*For treatment of congenital heartdiseases which are ductusarteriosus dependent45Alteplase 50 mgper vial InjectionB01AD02000P4001XXA*Thrombolytic treatment of acuteischaemic stroke.46AluminiumHydroxide 600mg TabletA02AB01250T1001XXADyspepsia, hyperphosphataemiaAcute promyelocytic leukaemiaDosageInduction: 45 mg/m2 dailyfor 30 - 90 days.Maintenance: 45 mg/m2daily for 2 weeks every 3months. Renal/or hepaticinsufficiency: 25mg/m2 dailyfor 30-90 days. Refer toprotocols0.25 - 0.5 mg 3 times daily(elderly or debilitated 0.25mg 2-3 times daily),increased if necessary to atotal dose of 3 mg/day. Notrecommended for children0.25 - 0.5 mg 3 times daily(elderly or debilitated 0.25mg 2-3 times daily),increased if necessary to atotal dose of 3 mg/day. Notrecommended for children0.25 - 0.5 mg 3 times daily(elderly or debilitated 0.25mg 2-3 times daily),increased if necessary to atotal dose of 3 mg/day. Notrecommended for children0.05 - 0.1 mcg/kg/min bycontinuous IV infusion, thendecreased to lowesteffective dose0.9 mg/kg (maximum of 90mg) infused over 60 minuteswith 10% of the total doseadministered as an initialintravenous bolus.Treatment must be startedas early as possible within4.5 hours after onset ofstroke symptoms and afterexclusion of intracranialhaemorrhage byappropriate imagingtechnique.600 mg- 1.2 g 4 times dailyand at bedtime or asrequired11 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)47Amantadine HCl100 mg CapsuleN04BB01110C1001XXBParkinson's disease48Amikacin 125mg/ml InjectionJ01GB06183P3003XXAInfections due to GB06183P3002XXAInfections due to susceptibleorganisms50Amiloride HCl 5mg &Hydrochlorothiazide 50 mg TabletC03EA01900T1001XXBi) Diuretic as an adjunct to themanagement of oedematous statesii) Hypertension51Amino AcidsInjectionB05BA01910P3001XXASource of amino acids in patientsneeding IV nutritionB05BA10910P3002XXASource of amino acids andelectrolytes in patients needing IVnutritionB05BA10910P3003XXASource of amino acids,carbohydrate and electrolytes inpatients needing IV nutrition5253Amino Acids withElectrolytesInjectionAmino Acids withGlucose withElectrolytesInjectionDosageInitial dose: 100 mg dailyand is increased to 100 mgtwice daily (not later than 4p.m.) after a week. Elderlyover 65 years: less than 100mg or 100 mg at intervals ofmore than 1 dayADULT: (IM or IV): 15mg/kg/day 8 - 12 hourly for7 - 10 days. Maximum: 1.5g/day. CHILD: 15 mg/kg/day8 - 12 hourly. Maximum: 1.5g/day. Neonates: Initialloading dose of 10 mg/kgfollowed by 7.5 mg/kg/day12 hourly. Maximum15mg/kg/dayADULT: (IM or IV): 15mg/kg/day 8 - 12 hourly for7 - 10 days. Maximum: 1.5g/day. CHILD: 15 mg/kg/day8 - 12 hourly. Maximum: 1.5g/day. Neonates: Initialloading dose of 10 mg/kgfollowed by 7.5 mg/kg/day12 hourly. Maximum15mg/kg/dayi) Initially 1 - 2 tab dailyadjusted according toresponse. Max: 4 tabs daily.ii) 1 -2 tabs daily as a singleor divided doseDose to be individualized.ADULT usually 500-2000 mlby IV. ADULT usualrequirement for amino acid:1-2 g/kg/dayDose to be individualized.ADULT usual requirementfor amino acid 1-2 g/kg/dayDose to be individualized.ADULT usual requirementfor amino acid 1-2 g/kg/day,carbohydrate 4-6 g/kg/day12 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)54Amino Acids,Glucose and Lipidwith ElectrolytesInjectionB05BA10910P3001XXASource of amino acids,carbohydrate, lipid and electrolytesin patients needing IV nutrition55Aminophylline 25mg/ml InjectionR03DA05000P3001XXBReversible airways obstruction,acute severe brochospasm56Amiodarone 200mg TabletC01BD01110T1001XXA*Arrhythmias57Amiodarone 50mg/ml InjectionC01BD01110P3001XXA*Arrhythmias when other drugs arecontraindicated or ineffectiveA*Treatment of psychoses,particularly acute or chronicschizophrenia disorderscharacterized by positivesymptoms(e.g. delusion,hallucinations, thought disorders)and/or negative symptoms(e.g.blunted emotions, emotional andsocial withdrawal) including whenthe negative symptomspredominate58Amisulpride 100mg TabletN05AL05000T1001XXDosageDose to be individualized.ADULT: 500 - 2000 ml dailygiven by IV. ADULT usualrequirement for amino acid1-2 g/kg/ day, carbohydrate4-6 g/kg/day, lipid 2-3g/kg/dayAdult: Loading dose: 5mg/kg (ideal body weight)or 250-500 mg (25 mg/ml)by slow injection or infusionover 20-30 min.Maintenance infusion dose:0.5 mg/kg/hr. Max rate: 25mg/min. Child: Loadingdose: same as adult dose.Maintenance dose: 6 mth-9yr: 1 mg/kg/hr and 10-16 yr:0.8 mg/kg/hr.200 mg 3 times daily for 1week, then reduced to 200mg twice daily for anotherweek. Maintenance dose,usually 200 mg daily or theminimum required tocontrol the arrhythmiaInitial infusion of 5mg/kg vialarge venous access over 20120 minutes with ECGmonitoring; subsequentinfusion given if necessaryaccording to response up toa maximum of 1.2 g in 24hoursPredominantly negativeepisodes: 50-300 mg oncedaily adjusted according tothe patient’s response.Mixed episodes withpositive and negativesymptoms: 400-800 mg/dayin 2 divided doses adjustedaccording to the patient’sresponse. Should be takenon an empty stomach(Preferably taken beforemeals)13 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategoryIndication(s)59Amisulpride 400mg TabletN05AL05000T1002XXA*Treatment of psychoses,particularly acute or chronicschizophrenia disorderscharacterized by positivesymptoms(e.g. delusion,hallucinations, thought disorders)and/or negative symptoms(e.g.blunted emotions, emotional andsocial withdrawal) including whenthe negative symptomspredominate60Amitriptyline HCl25 mg TabletN06AA09110T1001XXBDepression61Amlodipine 10mg and Valsartan160 mg TabletC09DB01935T1003XXA/KK62Amlodipine 10mg TabletC08CA01000T1002XXB63Amlodipine 5 mgand Valsartan160 mg TabletC09DB01935T1002XXA/KKEssential hypertension in patientswhose blood pressure is notadequately controlled bymonotherapyHypertensionEssential hypertension in patientswhose blood pressure is notadequately controlled bymonotherapyDosagePredominantly negativeepisodes: 50-300 mg oncedaily adjusted according tothe patient’s response.Mixed episodes withpositive and negativesymptoms: 400-800 mg/dayin 2 divided doses adjustedaccording to the patient’sresponse. Should be takenon an empty stomach(Preferably taken beforemeals)Initially 25mg 3 times a day.Maintenance: 25-100mgdaily in divided doses.Hospitalized patient:100mg/day &graduallyincrease to 200-300mg/day.ADOLESCENT and ELDERLY:initially 20-30mg/day individed doses w/ gradualincrements. CHILD under 16years are not recommendedDoses range fromamlodipine besylate 5mg/valsartan 160 mg toamlodipine besylate 10mg/valsartan 320 mgORALLY once daily, withdose titration occurringevery 1 to 2 weeks ifnecessary. MAX amlodipinebesylate 10 mg/valsartan320 mg5 mg once daily. Max: 10 mgonce dailyDoses range fromamlodipine besylate 5mg/valsartan 160 mg toamlodipine besylate 10mg/valsartan 320 mgORALLY once daily, withdose titration occurringevery 1 to 2 weeks ifnecessary. MAX amlodipinebesylate 10 mg/valsartan320 mg14 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic NameMDCCategory64Amlodipine 5 mgTabletC08CA01000T1001XXB65Amlodipinebesylate 10mg,valsartan 160mg,hydrochlorothiazide 12.5mg onTreatment of essentialhypertension. This fixedcombination drug is not indicatedfor the initial therapy ofhypertension.Dosage5 mg once daily. Max: 10 mgonce dailyOne tablet daily i) A patientwhose blood pressure is notadequately controlled ondual therapy withamlodipinebesylate/valsartan/HCTZ. ii)For convenience, patientsreceiving valsartan,amlodipine and HCTZ fromseperate tablets may beswitched to amlodipinebesylate/valsartan/HCTZcontaining the samecomponent dosses. Dosagemay be increased after 2weeks. The maximumantihypertensive effect ofamlodipinebesylate/valsartan/HCTZ isreached within 2 weeks ofchange in dose. Themaximum recommendeddose of amlodipinebesylate/valsartan/HCTZ is10/320/25 mg. It can betaken with or without food.It is recommended to take itwith some water.15 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.66Generic NameAmlodipinebesylate 10mg,valsartan 160mg,hydrochlorothiazide 25mg )Treatment of essentialhypertension. This fixedcombination drug is not indicatedfor the initial therapy ofhypertension.DosageOne tablet daily i) A patientwhose blood pressure is notadequately controlled ondual therapy withamlodipinebesylate/valsartan/HCTZ. ii)For convenience, patientsreceiving valsartan,amlodipine and HCTZ fromseperate tablets may beswitched to amlodipinebesylate/valsartan/HCTZcontaining the samecomponent dosses. Dosagemay be increased after 2weeks. The maximumantihypertensive effect ofamlodipinebesylate/valsartan/HCTZ isreached within 2 weeks ofchange in dose. Themaximum recommendeddose of amlodipinebesylate/valsartan/HCTZ is10/320/25 mg. It can betaken with or without food.It is recommended to take itwith some water.16 / 371
MINISTRY OF HEALTH MEDICINES FORMULARY - 2/2015No.Generic lorothiazide12.5mg tablet68AmlodipineCamsylate 5 mgand LosartanPotassium 100mg yIndication(s)A/KKTreatment of essentialhypertension. This fixedcombination drug is not indicatedfor the initial therapy ofhypertension.A/KKTreatment of essentialhypertension in adults patientswhose blood pressure is notadequately controlled on eithermonotherapyDosageOne tablet daily i) A patientwhose blood pressure is notadequately controlled ondual therapy withamlodipinebesylate/valsartan/HCTZ. ii)For convenience, patientsreceiving valsartan,amlodipine and HCTZ fromseperate tablets may beswitched to amlodipinebesylate/valsartan/HCTZcontaining the samecomponent dosses. Dosagemay be increased after 2weeks. The maximumantihypertensive effect ofamlodipinebesylate/valsartan/HCTZ isreached within 2 weeks ofchange in dose. Themaximum recommendeddose of amlodipinebesylate/valsartan/HCTZ is10/320/25 mg. It can betaken with or without food.It is recommended to take itwith some water.Amlodipine 5mg/losartan50mg OR amlodipine5mg/losartan 100mg orallyonce daily. MAXIMUMDOSE: amlodipine5mg/losartan 100mg. Nodosage adjustment in mildrenal impairment. Notrecommended in moderateto severe renal impairmentor in patients on dialysis.Not recommended inpatients who require lowerdose of losartan (25mg). Notrecommended in patients 18 years as safety andefficacy is not established
dose. iii) & iv) Crohn's disease & Ulcerative colitis: 160mg at week 0 (dose can be administered as four injections in one day or as two injections per day for two consecutive days) and 80mg at week 2. After induction treatment, the recommended maintenance dose is 40mg every other week via subcutaneous injection. 19 Adapalene 0.1% Cream .











