Transcription
TABLE OF CONTENTSBABS3041(Immunology)Term 1, 2020Course ManualSCHOOL OF BIOTECHNOLOGY AND BIOMOLECULAR SCIENCESFACULTY OF SCIENCE
COURSE INFORMATION . 5COURSE SUMMARY . 5COURSE AIMS . 5COURSE LEARNING OUTCOMES (ASSOCIATED ASSESSMENTS) . 5STRATEGIES AND APPROACHES TO LEARNING . 6TEXTBOOK . 6COURSE CONVENER AND LECTURERS . 7LECTURE OUTLINE . 8PRACTICAL CLASSES . 10LECTURE TIMETABLE AND LOCATION . 11PRACTICAL CLASS TIMETABLE . 12ASSESSMENTS . 13CONTINUOUS ASSESSMENT (50%) . 13FINAL EXAM (50%) . 13SPECIAL CONSIDERATIONAND FURTHER ASSESSMENT . 14SUPPLEMENTARY EXAMINATIONS . 15PLAGIARISM . 15LAB RULES & SAFETY . 16BIOLOGICAL HAZARDS . 17CHEMICAL HAZARDS . 17PHYSICAL HAZARDS . 17IN CASE OF EMERGENCY . 17SAFELY REMOVING GLOVES . 17HEALTH AND SAFETY PRECAUTIONS FOR ELECTRONIC DEVICESINCLUDING LAPTOP COMPUTERS AND MOBILE PHONES . 19RISK ASSESSMENTS . 19WEEK 2CELLS AND ORGANS OF THE IMMUNE SYSTEM 20AIMS . 20INTRODUCTION . 202
RISK ASSESSMENT . 20ACTIVITIES . 20PREPARATION AND STAINING A BLOOD SMEAR . 20RESULTS & WORKING . 23LYMPHOID ORGANS . 25WEEK 3TETANUS TUTORIAL . 26ACTIVITIES . 26TETANUS - THE BASIC INFORMATIOn. 27ANSWER THE FOLLOWING QUESTIONS . 28WEEK 4TUTORIAL: THE IMMUNE SYSTEM IN DIFFERENTSTAGES OF THE HUMAN LIFE . 34INTRODUCTION. 34ACTIVITIES . 34WEEK 5CELL VIABILITY AND COUNTING . 39AIMS . 39INTRODUCTION. 39RISK ASSESSMENTS . 39REAGENTS & EQUIPMENT . 39PROCEDURE . 39RESULTS & WORKING . 42QUESTIONS. 43WEEK 7ISOLATING & ACTIVATING SPLENOCYTES . 45AIMS . 45INTRODUCTION. 45RISK ASSESSMENT . 46PROCEDURES . 48CALCULATION OF CELL CONCENTRATIONS USED FOR YOUREXPERIMENTS . 51WEEK 8MEASUREMENT OF IL-2 USING AN ENZYMELINKED IMMUNOSORBENT ASSAY (ELISA) . 523
AIMS . 52INTRODUCTION . 52RISK ASSESSMENT. 52DESIGN OF ASSAY TEMPLATE . 53REAGENTS & EQUIPMENT . 53PROCEDURE . 54RESULTS & WORKING . 56QUESTIONS . 59QUALITY CONTROL. 60SENSITIVITY AND SPECIFICITY OF ELISA . 62WEEK 9FLOW CYTOMETRY . 63AIMS . 63ACTIVITIES . 63INTRODUCTORY MATERIALS OF FLOW CYTOMETRY . 63VIDEO TUTORIALS. 68COMMON PHENOTYPIC MARKERS OF HUMAN PBMCS . 68INTERPRETING CLINICAL DATA . 69DRAWING YOUR OWN DOT PLOTS. 73WEEK 10CLINICAL CASES . 74AIMS . 74INTRODUCTION . 74DIAGNOSTIC TESTS . 74CASE STUDIES. 78HLA TYPING . 894
COURSE INFORMATIONUnits of credit: 6Pre-requisite(s): (BIOC2101 or BIOC2181 and MICR2011)or (BIOC2101 or BIOC2181 and BABS2202).Teaching time: Term 1COURSE SUMMARYBABS3041 provides a broad and in-depth coverage of immunology. The courseis for students majoring in Medical Microbiology, Immunology, Biotechnology,Biomolecular Science, Medical Science and other areas related to human healthwho are interested in gaining knowledge in Immunology.BABS3041 consists of 24 one-hour lectures and 8 three-hour practical classes.The course will first introduce the multiple components of the immune system,their functions, interactions and regulations during immune responses. Then theapplied and clinical aspects of immunology will be introduced, including allergy,immunodeficiency, immune system and cancer, vaccination, autoimmunity,engineering antibodies, diagnostic immunology and immunological researchstrategies. The practical classes introduce students to critical immunologicaltechniques such as immune cell stimulation, immunological assays and flowcytometry. The course also introduces students to critical evaluation ofimmunological issues of community importance and literature.COURSE AIMSThis course provides students with a broad and in-depth understanding of theimmune system and its functions. It also introduces students to the applicationsrelated to the immune system and immunology research.COURSE LEARNING OUTCOMES (ASSOCIATED ASSESSMENTS)At the successful completion of this course, the students should be able to:1. Describe different components of the immune system and their responsesto infection and cancer (test 1, test 2 and final exam).2. Explain how immunological abnormalities may cause human diseases andhow immunological interventions may prevent and treat human diseases(test 1, test 2 and final exam).3. Apply diagnostic laboratory techniques to diagnose immunologicaldisorders (test 1, test 2 and final exam).4. Plan laboratory experiments and interpret experimental data on researchin immunology (the assignment and final exam).5. Critically evaluate scientific literature in immunology and immunologicalissues of community importance (the assignment and final exam).5
STRATEGIES AND APPROACHES TO LEARNINGBABS3041 uses Blended Learning strategy, combining face to face teaching withonline learning materials and activities. The course has been digitally uplifted.Lectures are delivered face to face and lecture notes are available on the courseMoodle website. All lectures are recorded and available via Moodle course site,providing flexibility and further assisting students’ learning. Practical classesconsist of laboratory-based experiments and computer-based classes. Other online learning activities and materials will be provided during the course.You can access Moodle site for this course via the UNSW website using yourUniPass.TEXTBOOKThe recommended textbook for this course: Janeway’s Immunobiology 9thEdition. Kenneth Murphy. 2017. Garland Science, Taylor & Francis Group,LLC.Other recommended book: Kuby Immunology 8th Edition.Freeman and Company.62019. W.H.
COURSE CONVENER AND LECTURERSDr. Li Zhang: Dr Zhang is the course convener and lecturer for BABS3041. DrZhang obtained her MB BS degree from Fudan University and PhD degree fromthe University of Cambridge. She is currently a Senior Lecturer at the Universityof New South Wales and has been the convener for this immunology course since2012. Dr Zhang has a long-standing interest in chronic inflammatory diseases.Her previous research was on autoimmune diseases. Since 2008, her group hasbeen research on Campylobacter species and their role in inflammatory boweldisease, the genomes and virulence of emerging gut bacterial pathogens, therelationship between gut microbiota and immunotherapy. Dr Zhang can becontacted at: Room 4106, Bioscience South E26. l.zhang@unsw.edu.auGuest lecturersProfessor Stuart Tangye: Professor Tangye is an immunologist from the GarvanInstitute of Medical Research. His research focuses on autoimmune disorders,immunodeficiency, inflammatory diseases and allergic diseases. ProfessorTangye delivers the two lectures on B cell and antibody and the two lectures onT cell functions. s.tangye@garvan.org.auDr Debbie Burnett: Dr Debbie Burnett is an Immunologist from the GarvanInstitute of Medical Research. Her research focus is understanding how theimmune system discriminates between self and foreign antigens and how thisrelates to pathogen defence and autoimmune disease. Dr Burnett delivers onelecture on antigen receptors and signalling. d.burnett@garvan.org.auAssociate Professor Stuart Turville: Associate Professor Turville is from theKirby Institute, UNSW. His group researches on the mechanisms of HIV spreadand using gene therapy to treat HIV infection. Associate Professor Turvilledelivers the two lectures on immunodeficiency. sturville@kirby.unsw.edu.au7
LECTURE OUTLINEOverview of the Immune System: Different components of the immune systemwill be introduced.Innate Immunity (two lectures): The innate immune system will be introducedin these two lectures. Key cells and molecules of the innate immune system willbe described.Adaptive Immunity: This lecture is an introduction to the adaptive immunesystem. Antigen, B cells, T cells, the clonal selection theory and immunologicalmemory will be introduced.B cells and Antibodies (two lectures): The structure and function of antibodieswill be outlined. The concept of specificity, affinity and affinity maturation willbe discussed. The development of B cells and their generation of the diverseantibody repertoire will be introduced.T cell Function (two lectures): The two lectures introduce various types of Tcells, their development and functions.Cytokines: The general nature of cytokines and the function of selectivecytokines will be introduced.Antigen Receptors and Signaling: This lecture introduces the recognition ofantigen by B cell and T cell receptors. Co-receptors and molecules of thedownstream signaling pathways will also be introduced.MHC and Antigen Presentation: The special way in which T cells interact withforeign antigen will be introduced. Students will learn about the MajorHistocompatibility Complex (MHC).Mucosal Immunity: Most infections begin at the mucosal surface and mucosalimmune system plays an important role in protecting the mucosal surface. Thespecific features and function of the mucosal immune system will be introduced.The Immune Responses to Infection (two lectures): Key defences againstdifferent types of infections will be outlined.Immune Evasion: Strategies that are used by microbes to evade host immuneresponse will be described.Immunodeficiency (two lectures): Students will be introduced to primaryimmunodeficiencies that affect different components of the immune system. HIV/AIDS will be the focus of the presentation on acquired immunodeficiency.Allergic Disease: Allergic disease is a serious problem in the modern world. Thislecture will introduce cells and molecules of the Type 1 hypersensitivity reaction.8
Vaccination: The history of vaccination will be introduced. Different types ofvaccines will be explained, and future challenges for vaccine development willbe outlined.Tolerance and Autoimmunity: The mechanisms by which self-reactivity of Bcells and T cells are either eliminated or controlled will be outlined, andautoimmunity will be discussed.Diagnostic immunology: This lecture will introduce some tests that are used inDiagnostic Immunology.Immune System and Cancer: In this lecture, you will learn cancers of theimmune system and the role of the immune system in controlling cancer.Engineering Antibodies: The engineering of antibodies is a huge industry. Theuse of antibodies as biopharmaceuticals and the approaches to antibodyengineering will be presented.Immunological Research Strategies: This lecture will describe severalstrategies that are used in the studies of immunology.9
PRACTICAL CLASSESATTENDANCE TO PRACTICAL CLASSES IS COMPULSORY exceptfor Week 4 tutorialPractical classes will be held on Tuesdays. The contents covered in the morningand afternoon class are same, you need to attend either morning or afternoonclass. You will be organized into groups and demonstrators will be assigned.The specific objectives of the practical classes are: To reinforce and better understand information delivered in lectures.To provide students with opportunities to explore techniques that arecommonly used in immunology research and diagnostic immunologyDevelop experimental and analysis skillsApply concepts and skills learned in this course to solve problems10
LECTURE TIMETABLE AND LOCATIONMon 5 -6 pm, Old Main Building 149(K-K15-149)Wed 1 -2 pm, Mathews Theatre D (K-D23-304)Thu 1 - 2 pm, Webster Theatre B (K-G15-290)LectureTopicDateWeek Lecturer123456Overview of the Immune SystemInnate Immunity 1Innate Immunity 2Adaptive ImmunityB cells and Antibodies 1B cells and Antibodies ZLZLZLZST1ST1789101112T cell Function 1T cell Function 2CytokinesAntigen Receptors and SignalingMHC and Antigen PresentationMucosal 44ST1ST1LZDBLZLZ13 Immune Responseto Bacterial Infection 16/3 Mon14 Immune Response to Viral Infection18/3 Wed15 Immune Evasion19/3 Thu555LZLZLZST2ST2LZLZLZLZWeek 6: Flexibility Week161718192021Secondary ImmunodeficiencyPrimary ImmunodeficiencyAllergic DiseaseVaccinationTolerance and AutoimmunityDiagnostic 888Public Holiday13/4 Mon915/4 Wed16/4 Thu20/4 Mon9 LZ9 LZ10 LZ22 Immune System and Cancer23 Engineering Antibodies24 Immunological ResearchLZ: Dr Li Zhang, ST1: Professor Stuart Tangye, DB: Dr Deborah Burnett,ST2: Associate Professor Stuart Turville11
PRACTICAL CLASS TIMETABLEMorning groups 10 am - 1 pm. E26 Teaching Lab 12 (K-E26 -1102)Afternoon groups 2 – 5 pm.E26 Teaching Lab 12 (K-E26 -1102)Week DatePractical class225/2 TueCells and tissues of the immunesystem33/3Tutorial: TetanusTueTest 1410/3 Tue517/3 TueTutorial: The Immune systemin different stages of the humanlifeCell viability and countingTest 2Week 6: Flexibility Week731/3TueIsolating & activatingsplenocytes87/4914/4 TueFlow cytometry1021/4Tutorial: Clinical casesTueTueMeasurement of IL-2 usingELISA12
ASSESSMENTSCONTINUOUS ASSESSMENT (50%)TEST1 (17.5%) and TEST2 (17.5%)There will be two tests, which will be held at the start of the practical classesin week 3 and week 5 respectively. Each test is worth 17.5% of your finalmark.Students who miss a test may have the chance to sit a supplementary test, onlyif they submit a medical certificate or other suitable documents to explain theirabsence to the course convener by the end of the week of the test. There willonly be ONE opportunity to sit the supplementary test, which will be held inthe week following the test.ASSIGNMENT (15%)This year’s assignment is related to week 7 and week 8 practical classes. Theassignment will be released on Moodle on 30th March. The deadline forsubmission of this assignment is 3pm, 16th April. There is 10% per daydeduction for late submissions. The assignment should be submittedelectronically to Moodle site. The hard copy must be submitted to theBABS/BEES/SOMS Student office.FINAL EXAM (50%)The final exam is worth 50%, which is scheduled within the exam period atthe end of the term. Further information regarding the final exam will beprovided in the last lecture.13
SPECIAL CONSIDERATIONAND FURTHER ASSESSMENTStudents who believe that their performance, either during the session or in theend of session exams, may have been affected by illness or other circumstancesmay apply for special consideration. Applications can be made for compulsoryclass absences such as (laboratories and tutorials), in-session assessments tasks,and final examinations. Students must make a formal application for SpecialConsideration for the course/s affected as soon as practicable after the problemoccurs and within three working days of the assessment to which it refers.Students should consult the “Special Consideration” section of Moodle forspecific instructions related to each BABS course they are studying. Furthergeneral information on special consideration can also be found n.HOW TO APPLY FOR SPECIAL CONSIDERATIONApplications must be made via Online Services in myUNSW. You must obtainand attach Third Party documentation before submitting the application.Failure to do so will result in the application being rejected. Log intomyUNSW and go to My Student Profile tab My Student Services channel Online Services Special Consideration. After applying online, studentsmust also verify supporting their documentation by submitting to UNSW StudentCentral: Originals or certified copies of your supporting documentation (StudentCentral can certify your original documents), and A completed Professional Authority form (pdf - download here).The supporting documentation must be submitted to Student Central forverification within three working days of the assessment or the period coveredby the supporting documentation. Applications which are not verified will berejected.Students will be contacted via the online special consideration system as to theoutcome of their application. Students will be notified via their official universityemail once an outcome has been recorded.14
SUPPLEMENTARY EXAMINATIONSSUPPLEMENTARY EXAMINATIONS:The University does not give deferred examinations. However, supplementaryexams may be given to those students who were absent from the final examsthrough illness or misadventure. Special Consideration applications for finalexams and in-session tests will only be considered after the final exam periodwhen lists of students sitting supplementary exams/tests for each course aredetermined at School Assessment Review Group Meetings. Students will benotified via the online special consideration system as to the outcome of theirapplication. It is the responsibility of all students to regularly consult theirofficial student email accounts and myUNSW in order to ascertain whetheror not they have been granted further assessment.The supplementary exam period for T1 2020:25 May –29 MaySupplementary exams will NOT be offered on any alternative dates.PLAGIARISMPlagiarism is using the words or ideas of others and presenting them as your own.It can take many forms, from deliberately cheating to accidentally copying froma source without proper acknowledgement. If any student is caught plagiarizingin this course. they will receive no marks for that course task. For furtherinformation on plagiarism, the university Learning Centre has substantialinformation on the subject including material on how to avoid plagiarism.Information can be found at: www.lc.unsw.edu.au/plagiarism.15
LAB RULES & SAFETYBiological laboratories are inherently dangerous places and it is the responsibilityof everyone who works in the laboratory to ensure that the risks are minimized.It is your responsibility to ensure that you come to labs prepared and work in amanner that will not put yourself or others in danger. Risks related to eachpractical class are outlined individually. Some general laboratory rules areoutlined below. Wear a fastened lab coat.Wear disposable gloves when handling samples and other experimentalmaterials and dispose the gloves if they are contaminated.Dispose all materials in the appropriate bins. All contaminated waste mustgo in the biohazard bags provided. Broken glass must go in the brokenglass bin and anything sharp in the sharps bin.Wear fully enclosed shoes, preferably made of a sturdy material.Keep all hair tied back.Move calmly and slowly around the lab.Keep only what is necessary on your desk. All other things should be keptin the cabinets provided.Wipe down benches, before and after the practical class.Act maturely and responsibly.Do not eat, drink, apply makeup or chew gum (or pens). Any food or drinkyou have must be stored inside your bag at all times during the lab.Do not invite any one into the lab. Only the people who are meant to bein the lab should be there.Keep all experimental reagents and equipment covered. Never leavethings such as pipette tips etc lying around uncovered unnecessarily.Never attempt to recap a needle.Label all samples with your name, date, demonstrator and what kind ofsample it is.Never handle ‘clean items’ such as your bag whilst wearing gloves. Evenif you think your gloves are clean, they may not be.Wear safety goggles when handling things that may splash into your eyes.16
BIOLOGICAL HAZARDS All biological samples are potentially harmful if ingested or exposed tobody surfaces. Ensure that you wash your hands thoroughly with an antimicrobial detergent and dry them thoroughly before leaving the lab. All specimens of cells, tissues or body fluids from humans or animals arepotential sources of infection. Every effort is made to ensure that anyspecimens you receive are not infectious. However, the use of universalprecautions when handling specimens is essential. The main principle ofuniversal precautions is to assume everything is a source of infection. Always wear gloves, a lab coat, closed toe shoes and when there is achance of splashing, safety goggles. keep your lab coat in a plastic bagafter classes to avoid spreading any potential contamination.CHEMICAL HAZARDSMost buffers and media used in the course are not or low hazards at theconcentrations used, however all chemicals should be considered potentiallyhazardous.PHYSICAL HAZARDSCommon sense precautions must be observed when dealing with heat sourcessuch as Bunsen burners water baths as well as sharp objects.IN CASE OF EMERGENCY If there is an accident with a biological sample, try to mop up what youcan and call a fellow student to get a demonstrator to help. Do not moveyourself in case you spread the contamination further. If there is a fire, remove yourself from immediate danger and call someonein authority to help. Call a demonstrator immediately if anything happens. Do not try and dealwith the situation yourself. If you splash something in your eye, flush it out at an eye stationimmediately and ask someone to call a demonstrator for help. If there is a medical emergency call a demonstrator for help, and then tryand ascertain what caused the emergency (e.g. leaking gas tap, exposedpower cable) and rectify the situation if you think you can do so safely. Ifnot, wait for your demonstrator.SAFELY REMOVING GLOVESIn the immunology lab, we often wear gloves to protect ourselves from thesubstances that we are working with. However, this only works if we can safelyremove those gloves without contaminating our hands (photos used with17
permission from the University of Maryland, Department of EnvironmentalSafety).Pinch one glove in the palm and pull towardsthe ends of the fingers until the gloves foldsover.Keep pulling so that the fingers are inverted.Scrunch the removed glove in the palm of theother gloved hand.Now, slide your index finger underneath theremaining glove.Pull the glove down over the fist.18
Touch only the inside of the gloves and dropinto the appropriate container. Wash handsthoroughly.HEALTH AND SAFETY PRECAUTIONS FOR ELECTRONIC DEVICESINCLUDING LAPTOP COMPUTERS AND MOBILE PHONESMobile phones: For your own safety when using your mobile phone in classplease ensure it is placed in a plastic zip lock bag. Every student will be providedwith a zip lock bag in their first practical class which they should continue to useduring the session (keep it with your lab book). If you misplace or loose this bagyou will be expected to provide your own zip lock bag.Computers and tablets: this applies to either your own device or those suppliedby the school. Please cut a section of benchcoat (this will be provided in the labclass) and place your device on this on the lab bench to separate it from your otherlaboratory work. DO NOT wear gloves when using these devices. If the lab isbeing used as a dedicated DRY LAB (please check signage on the door) you willnot be required to do this as the benches will be cleaned and decontaminatedbefore your class.RISK ASSESSMENTSYou will be required to complete Risk Assessment forms before wet laboratoryclasses.19
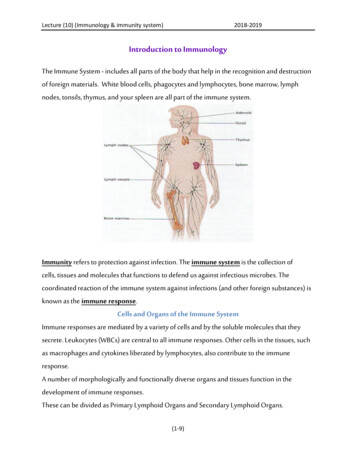
WEEK 2CELLS AND ORGANS OF THE IMMUNE SYSTEMAIMS To familiarize students with the preparation of a stained blood smear To develop your ability to identify different types of leucocytes in theblood To better understand the anatomy of the immune systemINTRODUCTIONIn this practical class, you will prepare a blood smear and use it to learn how toidentify the major cell types of the blood. You will also perform a differential cellcounting to quantify the relative proportions of different immune cell types in theblood. Differential cell counting is a standard clinical test, in many clinicalconditions the relative proportions of different cell types change.You then examine the structure of some lymphoid organs, which will help you tobetter understand the anatomy of the immune system.At the end of this practical class, you should have a good understanding of thecommon immune cells and the anatomy of lymph nodes, the spleen and thethymus.RISK ASSESSMENTThis practical class involves the use of horse blood, which is commerciallypurchased. However, you should treat all blood samples as potentiallyinfectious. You must also remember that there may be unidentifiable pathogensin the blood. These issues must guide your risk assessment and the proceduresyou follow in the practical class to minimize these risks.ACTIVITIES Preparation of a blood smearStaining the blood smearDifferential blood leukocyte countExamination of the structure of lymphoid organsPREPARATION AND STAINING A BLOOD SMEARREAGENTS & EQUIPMENT Horse bloodGlass microscope slidesBenchkote paperBiohazard waste disposal bagBiohazard sharps disposal bin20 p
The recommended textbook for this course: Janeway's Immunobiology 9th Edition. Kenneth Murphy. 2017. Garland Science, Taylor & Francis Group, LLC. Other recommended book: Kuby Immunology 8th Edition. 2019. W.H. Freeman and Company.