Transcription
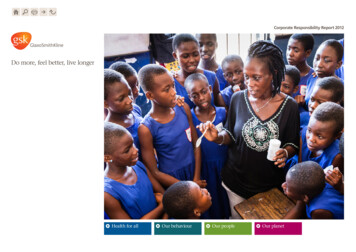
Do more, feel better, live longerGlaxoSmithKlineAnnual Report 2010
Contents0810121418192021222934414753Governance and remuneration P58–P101Governance and remunerationOur BoardOur Corporate Executive TeamGovernance and policyDialogue with shareholdersInternal control frameworkCommittee reportsRemuneration policyDirector terms and conditionsDirector and Senior Management remunerationDirectors’ interestsDirectors’ interests in contracts58606469717484919496101Financial statements P102–P191Financial statementsDirectors’ statement of responsibilitiesIndependent Auditors’ reportFinancial statementsNotes to the financial statementsFinancial statements of GlaxoSmithKline plcprepared under UK GAAPShareholder information P192–P212Business review P08–P57Business review2010 Performance overviewResearch and developmentPipeline summaryProducts, competition and intellectual propertyRegulationManufacturing and supplyWorld marketGSK sales performanceSegment reviewsResponsible businessFinancial review 2010Financial position and resourcesFinancial review 2009Risk factorsShareholder informationQuarterly trendFive year recordProduct development pipelineShare price and dividendsNature of trading marketAnnual General MeetingInvestor relations and RegistrarTaxation information for shareholdersGlossary of termsIndexBusiness reviewThis discusses our financial and non-financial activities,resources, development and performance during 2010and outlines the factors, including the trends and theprincipal risks and uncertainties, which are likely toaffect future development.Governance and remunerationThis discusses our management structures andgovernance procedures. It also sets out theremuneration policies operated for our Directors andCorporate Executive Team members.Financial statementsThe financial statements provide a summary of theGroup’s financial performance throughout 2010 and itsposition as at 31st December 2010. The consolidatedfinancial statements are prepared in accordance withIFRS as adopted by the European Union and also IFRS asissued by the International Accounting Standards Board.Shareholder informationThis includes the full product development pipeline anddiscusses shareholder return in the form of dividendsand share price 11212Underlying sales growth excludes pandemic products,Avandia and Valtrex. See page 21.CER% represents growth at constant exchange rates.Sterling % or % represents growth at actual exchangerates. See page 21.The calculation of results before major restructuringis described in Note 1 to the financial statements,‘Presentation of the financial statements’.GSK Annual Report 2010
01We exist to improve thequality of human life byenabling people to do more,feel better and live longer.We work by respecting people,maintaining our focus on the patientand consumer whilst operating withboth integrity and transparency.We are looking to deliver shareholdervalue through growth of a diversifiedand global business, by deliveringmore products of value, simplifyingour operating model and by runningour business responsibly.What follows is our report toshareholders for 2010. Progress wehave made in the year can also beseen by visiting our website:www.gsk.com/corporatereportingNotice regarding limitations on Director Liability under English LawUnder the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Report of the Directors contained on pages 8 to101. Under English law the Directors would be liable to the company, but not to any third party, if the Report of the Directors contains errors as a result of recklessness or knowingmisstatement or dishonest concealment of a material fact, but would not otherwise be liable.Report of the DirectorsPages 8 to 101 inclusive comprise the Report of the Directors that has been drawn up and presented in accordance with and in reliance upon English company law and the liabilitiesof the Directors in connection with that report shall be subject to the limitations and restrictions provided by such law.WebsiteGlaxoSmithKline’s website www.gsk.com gives additional information on the Group. Notwithstanding the references we make in this Annual Report to GlaxoSmithKline’swebsite, none of the information made available on the website constitutes part of this Annual Report or shall be deemed to be incorporated by reference herein.Cautionary statement regarding forward-looking statementsThe Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statementsmade, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations orforecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’,‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’ and other words and terms of similar meaning in connection with any discussion of future operating or financialperformance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current andanticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. The Group undertakes no obligation to update anyforward-looking statements, whether as a result of new information, future events or otherwise.Forward-looking statements involve inherent risks and uncertainties. The Group cautions investors that a number of important factors, including those in this document, couldcause actual results to differ materially from those contained in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Risk factors’on pages 53 to 57 of this Annual Report.GSK Annual Report 2010
02GSK at a glanceWe are one of the world’s leadingresearch-based pharmaceutical andhealthcare companies. We arecommitted to improving the qualityof human life by enabling peopleto do more, feel better and live longer.Our 2010 numbers 28.4bn32.1pTurnoverEarnings per share53.9p65pEarnings per share beforemajor restructuringHow we do itGSK has focused its business on the delivery of three strategicpriorities, which aim to increase growth, reduce risk andimprove GSK’s long-term financial performance:Dividend per shareGroup sales Grow a diversified global business1US Pharmaceuticals: 7.6bn Deliver more products of value2Europe Pharmaceuticals: 6.5bn3Consumer Healthcare: 5.0bn4Emerging MarketsPharmaceuticals: 3.6bn5Asia Pacific/JapanPharmaceuticals: 3.1bn6ViiV Healthcare: 1.6bn Simplify GSK’s operating modelWhere we do itGSK is a global organisation with offices in over 100 countriesand major research centres in the UK, USA, Belgium and China.Our shares are listed on the London and New York StockExchanges and our corporate head office is in Brentford, UK.67154237Research & developmentOther: 1.0bnConsumer Healthcarec.30 3.96bn 20%No.1A peer-leadingpipeline with around30 late-stage assets.In 2010, we spent 3.96bnin R&D before majorrestructuring, or 14%of our total sales.Growth of Horlicksin India in 2010.Sensodyne has been theworld’s fastest growingtoothpaste brand overthe last 5 years.We are one of the world’sbiggest investors in R&D and arethe biggest private sector funderof R&D in the UK.1014%c.1bn210 new compounds andvaccines starting phase IIIclinical trials since the startof 2010.We are committed toimproving returns in R&D,aiming to increase ourestimated returnon investment inthis area to 14%.Units of Lucozade,Ribena and Horlicksmanufactured inthe UK every year.New Consumer HealthcareResearch and Innovationcentres opened in Chinaand India.VaccinesEmerging markets1.4bn24%Doses of our vaccinessupplied to 179 countriesaround the world in 2010.Of total GSK turnoverfrom emerging markets,by the broader definition(Pharmaceutical andConsumer Healthcareturnover in all marketsexcluding USA, WesternEurope, Canada, Japan,Australia and New Zealand).GSK Annual Report 2010
03GSK at a glance24116 3532 3237333722Business review P08–P57A global company22245625 463232Sites with over100 employees:BiologicalsCorporateConsumer HealthcareGMSPharmaceuticalsResearch and Development5%3Share of worldpharmaceutical market.(Source: IMS Health)Leading presence inConsumer Healthcareglobal categories: OTC,Oral Care, Nutritionals.GSK’s business modelResponsible businessA balanced, synergistic business, with multiple growth driverssupporting a core pharmaceutical R&D operation.Malaria vaccinePotentially the first malaria vaccine with phase III trials ongoing in7 African countries.300 millionCommitment to supply 300m doses of Synflorix at a reduced priceto developing countries over the next decade through the AMCfinancing mechanism.CorePharmaceuticalR&DLeaderGSK ranked first in both Access to Medicine Indexes in 2008and 2010.2050Target date for value chain, from raw materials to product disposal,to be carbon neutral.To find out more visit us atwww.gsk.com/corporatereportingGSK Annual Report 2010Shareholder information P192–P2125-year commitmentTo treat school age children in Africa at risk of intestinal worms.Financial statements P102–P19196,500Employees.Governance and remuneration P58–P1012
04Chairman & CEO summaryDear ShareholderOver the last two and a half years we have been implementing astrategy to transform our business model to address the significantchallenges our industry faces as payers search for ever more costeffective healthcare, and demand escalates for new and bettermedicines. This is being done with the direct aim of enhancing returnsto our shareholders and improving the lives of patients and consumers.To achieve this we have substantially re-engineered GSK’s businessthrough major restructuring and a more rigorous approach tocapital allocation. The effects of these changes in 2010 weremasked to some degree by specific events. Reported sales, forexample, were impacted by generic competition to Valtrex, andreduced sales from Avandia and pandemic related products.Meanwhile earnings were impacted by the significant charge wetook to help resolve long-standing legal matters. This belies thegood progress we have made to execute our strategy and whichis evident in diversified underlying sales growth and the increasingpotential of our pipeline. We believe GSK is becoming a morebalanced, synergistic business with a lower risk profile and theoption for significant potential upside from the pipeline.Sir Christopher GentChairmanGSK Annual Report 2010GSK is also a business built on strong values and a deepcommitment to operating with integrity. In 2010 we have takenfurther steps to make our company more responsive, more flexibleand more open to society’s expectations.Increasing returns to shareholdersIn 2010 we were able to fund returns to shareholders, bolt-onacquisitions and the significant increase in legal settlements whilstreducing net debt by 0.6 billion.Adjusted 2010 net cash inflow before legal matters was 8.8billion, up 9%. Cash outflow for legal settlements was 2 billion.GSK remains financially very strong. We increased our dividend by7% to 65p in 2010 and our priority is to deliver further growth inthe dividend. Since 2005, dividends have increased each year withaverage growth of 8% over the five-year period. We havealso started a new long-term share buy-back programme toenhance returns to shareholders, with buy-backs of 1-2 billionexpected in 2011.Andrew WittyChief Executive Officer
05Chairman & CEO summaryOperating a values-based business with integrityContinuing to run our business in a responsible way is also centralto the changes we have made at GSK.This is helping to reduce GSK’s dependency on sales of productsgenerated in ‘white pills/western markets’†. Sales from thesemarkets and products have decreased from 40% in 2007, to 25%in 2010. Over time, this should help to reduce the adverse impactof patent expirations on the Group.Improving the environmental sustainability of our business is alsoa priority and we have launched a new set of ambitious targets.Our goal is to reduce the environmental impact of our whole valuechain, from raw materials to product disposal, and to be carbonneutral by 2050.Delivering diversified underlying sales growthIn 2010, reported sales fell 1%, impacted by the continuedeffect of generic competition to Valtrex, the rapid loss of salesof Avandia following regulatory decisions in the Autumn and adifficult comparison with the prior year which included significantsales of pandemic products.We are continuing to work towards resolving a number of longstanding legal matters. There is no doubt that the scale of legalprovisioning that has been required is significant. However, wecontinue to believe that it is in the Group’s best interests to resolvethis inherent unpredictability and reduce GSK’s overall litigationexposure. These legal cases underline just how important it is forus to be led by our values in everything we do.However, underlying sales growth (sales excluding these 3factors) was up 4.5%. This growth was achieved despite theongoing impacts of US healthcare reform and EU governmentausterity measures and is testament to the strength of the rest ofour portfolio.Increasing pipeline potentialReforming R&D to improve returns on investment has been a keyelement of the strategy we are implementing. We saw furtherevidence that this strategy is making progress during 2010.GSK now has a peer-leading portfolio of around 30 opportunitiesin phase III and registration. This portfolio is diverse with 5biopharmaceuticals and 5 vaccines in addition to NCEs. It is alsohighly innovative with more than 20 assets not currently availablefor any indication. One such asset – Benlysta – is potentially thefirst new treatment for lupus in 50 years and is currently beingconsidered for approval by regulators in the USA and Europe.We would like to thank Julian for his dedicated service to GSK asCFO and a member of the Board over the last six years – his integrity,diligence and outstanding technical ability have ensured that GSK hasremained financially strong during a period of significant economicturmoil. Simon’s appointment as CFO will bring valuable newexperience and capability to support us in implementing our strategy.ConclusionThere is no doubt that our operating environment remainschallenging and that the pharmaceutical industry is undergoinga period of intense change. However, we believe that GSK is wellplaced to succeed in this environment.We have made fundamental changes to how we allocate ourR&D expenditure, directing it to our late stage pipeline; reducingcost and risk through externalising parts of early-stage discovery;dismantling infrastructure; and terminating development in areaswith low financial and scientific return. Our target remains todeliver a rate of return for GSK’s R&D of around 14%. We arethe only pharmaceutical company to have explicitly set such achallenging target.Sir Christopher GentChairmanAndrew WittyChief Executive Officer† See page 21.GSK Annual Report 2010Shareholder information P192–P212Our journey to create a more balanced, synergistic businesswith increasing pipeline potential is progressing well and inImportantly, we are delivering sustained progress, with 10 NCEs and accomplishing this we would like to recognise the significantcontribution of our employees and our many partners. We remainnew vaccines entering phase III since the start of 2010. By the endconfident that we can generate increased value for shareholdersof 2012, we expect phase III data on around 15 assets, includingas well as deliver better outcomes to patients and consumers.potential new treatments for type 1 and 2 diabetes, several rarediseases and multiple cancer types.Financial statements P102–P191In 2011, we expect underlying sales momentum to continue andtranslate into sustainable reported growth in 2012.Changes to the BoardIn September we announced that Julian Heslop will retire as CFOat the end of March and be replaced by Simon Dingemans, whojoined the company as CFO-designate in January 2011.Governance and remuneration P58–P101We have taken cost out from lower returning activities andreinvested it in key growth areas such as Emerging Markets,Vaccines and Consumer Healthcare. 2010 reported sales for thesebusinesses were up 22%, 15% and 5% respectively.In 2010, we continued progress in our significant commitment towork on neglected tropical diseases. Our candidate malaria vaccineis progressing through phase III trials in Africa. If all goes well, thiswill be the first ever vaccine against malaria, with the potentialto save the lives of millions of children and infants in Africa. Wealso announced that we will donate enough of our albendazolemedicine to protect all school-aged children in Africa againstintestinal worms. Intestinal worms cause more ill health inschool-aged children than any other infection, so this will havea major positive health impact.Reinvestment of costs saved through our restructuring programmehas enabled us to diversify and strengthen GSK’s sales base. Todate, 1.7 billion of cost has been extracted from the business andwe are on track to deliver 2.2 billion of annual savings by 2012.Business review P08–P57Continuing focus on return on investmentOur drive for change, and to improve returns on investmentthrough restructuring and effective capital allocation, continued tomake progress during the year.
06Discover the world of GSKWe have chosen ten case studies from2010 that demonstrate the progress wehave made against our strategic priorities.Each of these stories can be viewed onlinewww.gsk.com/corporatereporting
07Our strategyGrow a diversified global businessOur plansWe are diversifying our business to create a more balanced product portfolio and move awayfrom a reliance on traditional ‘white pills/western markets’*. Sales generated from thesemarkets and products have decreased from 40% in 2007, to 25% in 2010. Over time thisshould help to reduce the adverse impact of patent expirations on the Group. Drive growth in the pharmaceuticalbusiness in our core marketsWe expect to generate future sales growth by strengthening our core pharmaceuticals businessand supplementing it with increased investment in growth areas such as Emerging Markets,vaccines, Japan, dermatology and Consumer Healthcare. Sales in Emerging Markets were up22%, vaccines up 15%, Japan up 14%, dermatology up 6% (on a pro-forma basis excluding2010 acquisitions) and Consumer Healthcare up 5% for 2010. Expand our business in JapanDeliver more products of valueOur plansWith the aim of sustaining an industry-leading pipeline of products that deliver value forhealthcare providers, we have been focusing on improving rates of return and delivering thebest science in our R&D organisation. This has required a multi-faceted approach. For examplewe have increased the level of externalisation of our research, taken difficult decisions aroundpipeline progressions and focused on disease areas where we believe the prospects forsuccessful registration and launch of differentiated medicines are greater. Focus on the best science Fulfil the potential of EmergingMarkets Build our leadership in dermatology Diversify through externalisation Re-personalise R&D Focus on return on investmentOur plansAs our business continues to change shape, it is essential that we transform the operatingmodel to reduce complexities, improve efficiencies and reduce cost. Through our globalrestructuring programme, we have removed 1.7 billion of cost since 2008 and are on trackto deliver our target of 2.2 billion of annual savings by 2012. These savings have beenextracted from our developed country sales and marketing, support functions, R&D andmanufacturing infrastructure and reinvested in higher returning activities such as EmergingMarkets, vaccines and Consumer Healthcare. Evolve our commercial model Re-shape manufacturing Streamline our processes Reduce working capitalOutlookWhilst our operating environment remains challenging, we have made significant progressthrough restructuring and a rigorous returns-based approach to capital allocation. We expectunderlying sales momentum (sales excluding Valtrex, Avandia and pandemic related products)to continue in 2011 and to translate into reported growth in 2012 at constant exchange rates,despite further anticipated pricing reductions in the USA and Europe.Shareholder information P192–P212The US patent for compositions containing the combination of active substances in Seretide/Advair expired during 2010, but various patents over the Diskus delivery device exist in the USAfor a number of years up to 2016. The outlook for the timing and impact of entry of ‘follow-on’competition is uncertain. GSK has not been notified of any acceptance by the US FDA of anapplication for a ‘follow-on’ product that refers to Seretide/Advair and contains the same activeingredients (as would be expected to precede the introduction of such a product), and is not ableto predict when this may occur or when any such ‘follow-on’ product may enter the US market.Other products may experience generic competition in advance of the stated patent expiry as aresult of settlement of patent proceedings. See Note 44, ‘Legal proceedings’, pages 178 to 185.Financial statements P102–P191Simplifying the operating modelGovernance and remuneration P58–P101 Grow the vaccines and ConsumerHealthcare businessesWe have one of the largest and most diverse development pipelines in the industry withapproximately 30 late-stage assets. The vast majority of these programmes address unmetmedical need and importantly nearly two-thirds are new chemical entities or new vaccines.GSK has a peer-leading development pipeline, with over 20 assets not currently on the marketfor any indication. By the end of 2012, we expect Phase III data on around 15 additional assets.With improvements in our net debt position, we are increasing returns to shareholders.We increased GSK’s dividend in 2010 and our priority is to deliver further growth in the dividend.We also have commenced a new long-term share buy-back programme.We remain confident that we can generate increased value for shareholders as well as deliverbetter outcomes to patients and consumers.* See page 21.Business review P08–P57Since 2008, we have focused our business around the deliveryof three strategic priorities, which aim to increase growth,reduce risk and improve our long-term financial performance:GSK Annual Report 2010
08Business review P08–P572010 performance overviewOur strategiesOur measuresOur progress in 2010We have focused the businessaround the delivery of threestrategic priorities.We use a number of measuresto track our progress againstthe strategic priorities over themedium to long term. Theseinclude the following:We made good progress during the year, with a numberof notable successes:Grow a diversifiedglobal business Performance of corepharmaceuticals andvaccines businesses Excluding pandemic products, Avandia and Valtrex,underlying pharmaceutical (including vaccines) sales*were 21.1 billion and grew 4% in the year. Diversification of sales Sales from ‘white pills/western markets’† fell from 40% ofturnover in 2007 to 25% in 2010. Contribution of EmergingMarkets to our overallsales and growth Sales in our Emerging Markets pharmaceutical businessgrew by 22% to more than 3.6 billion and now represent15% of pharmaceutical turnover. Growth of ConsumerHealthcare business Sales in our Consumer Healthcare business grew by 5% to 5.0 billion and now represent 17.6% of Group turnover. Build our leadership positionin dermatology Dermatology sales grew on a pro-forma basis (excluding2010 acquisitions) by approximately 6% to nearly 1.1 billion, representing nearly 4% of Group turnover. Expansion of Japanese business Sales in GSK Japan grew 14% to nearly 2.0 billion.Broadening and balancing ourportfolio and moving away froma reliance on ‘white pills/westernmarkets’†. We received approvals for four new compounds. Build biopharmaceutical portfolio Arzerra recorded sales of 26 million on its first full year onthe US market and was launched in Europe. Benlysta filedfor approval in both the USA and Europe.Deliver moreproducts of value Contribution to sales ofnew products New products launched since 2007 (excluding flupandemic vaccines) grew 36% and contributed 7% ofpharmaceutical sales in 2010.Transforming R&D to ensurewe not only deliver the currentpipeline but are also able tosustain the flow of productsfor years to come. Number of reimbursable productapprovals and filings We received six product approvals in the USA and EUsince the start of 2010.Simplifying theoperating modelSimplifying our operating modelto ensure that it is fit for purposeand able to support our businessin the most cost efficient way. Seven assets are currently filed with regulators. Sustaining late-stage pipeline We maintained around 30 assets in phase III andregistration, with ten new chemical entities and newvaccines entering phase III since the start of 2010. Enhanced R&D productivity andincreased externalisation forDrug Discovery Our objective is to increase our estimated rate of return forR&D from around 11% to 14%. Delivery of major restructuringprogramme We have achieved annual cost savings of 1.7 billion andremain on track to reach 2.2 billion of annualised savingsby 2012. Reduce working capital Working capital reduced by 1.3 billion in 2010 (including 600 million of cash from lower pandemic receivables).* The calculation of underlying sales growth is described on page 21.† See page 21. During 2010 we signed eight new collaborations toincrease the external nature of our discovery, giving54 external discovery engines to complement our 38Discovery Performance Units.
092010 performance overviewTurnoverCER growth %‡ 6In 2010, reported sales were down1% but underlying sales growth (salesexcluding pandemic products, Avandiaand Valtrex) was 4.5%.Business review P08–P57Key performance indicatorsEarnings per share before major 071099.11995.52006Earnings per share in 2010 was adverselyimpacted by legal costs of 4,001 million(2009 – 591 million). Excluding legalcosts, EPS before major restructuring was120.7 pence, 11% down on 2009.Free cash flow 5Total shareholder return150Financial statements P102–P1912008The reduced level of free cash flowin 2010 reflected the higher legalsettlements in the year. Free cashflow before legal settlements was 6,533 million (2009 – 5,508 million).Governance and remuneration P58–P101CER growth %#pence125100Shareholder information /0931/12/10GlaxoSmithKline Total Return IndexGlaxoSmithKline Pharma Peers Return Index ‡FTSE 100 Total Return Index‡This index includes Abbott Labs, Amgen, AstraZeneca, Bristol Myers Squibb,Eli Lilly, Johnson & Johnson, Merck, Novartis, Pfizer, Roche Holdings andSanofi-Aventis.Reflects 4bn legal charge.# The calculation of CER growth is described on page 21.* The calculation of results before major restructuring is described in Note 1 to thefinancial statements, ‘Presentation of the financial statements’. The calculation of free cash flow is described on page 44.GSK Annual Report 2010
10Business review P08–P57Research and developmentResearch and development – PharmaceuticalsGSK R&D has built one of the strongest, broadest pipelines ofpotential new medicines in the industry. We believe the pipelinehas the potential to deliver value to patients and payers andimprove rates of financial return on our R&D investment.Appropriately progressing our pipeline products safely andefficiently to deliver innovative new medicines for patients is theprimary goal of our R&D function.The development of new products typically is a long, expensiveand uncertain process, and it is not possible to predict whichcompounds in development will succeed or fail. The risks inherentin the R&D process are described more fully in the ‘Risk factors’section, under ‘Risk that R&D will not deliver commerciallysuccessful new products’.GSK allocates its R&D investment with reference to the potentialreturns available from its target therapeutic markets and thetechnical and commercial risks associated with products inthe pipeline. Those factors are reviewed at each phase of thedevelopment process and are central in the decision to proceed tothe next stage. Costs incurred at each stage are carefully managedto maximise the likely future return consistent with the Group’soverall objective of increasing its IRR from its R&D activities fromits current level, estimated in 2009 to be around 11%, to 14%.The returns generated are, however, primarily determined by theeventual commercial impact of new products as they achieveregulatory approval and are launched.This projected rate of return includes products launched from1st January 2007 and compounds in phases IIb and III of thedevelopment process. The calculation is based on actual salesfrom 2007 to 2009 and forecast sales for the relevant productsup to 2030, adjusted to reflect expected failure rates, which arebroadly in line with standard industry failure rates. The cost baseused in this calculation comprises an estimate of attributableR&D costs and actual and projected milestone payments whereappropriate. Estimated profit margins, capital investment andworking capital requirements are factored into the calculation,based on our historical performance.Details of the full product development pipeline, made up of bothpharmaceutical and vac
feel better and live longer. We work by respecting people, maintaining our focus on the patient and consumer whilst operating with both integrity and transparency. We are looking to deliver shareholder value through growth of a diversified and global business, by delivering more products