Transcription
Do more, feel better, live longerAnnual Report 2012
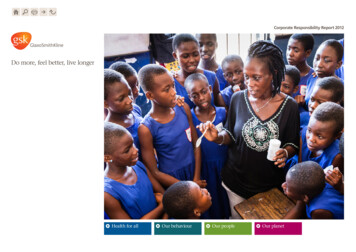
Our missionAt GSK our mission is toimprove the quality ofhuman life by enablingpeople to do more, feelbetter and live longer.Notice regarding limitations onDirector Liability under English LawUnder the UK Companies Act 2006, a safe harbour limitsthe liability of Directors in respect of statements in andomissions from the Report of the Directors containedon pages 1-136 and 239-244 which includes a businessreview on pages 1 to 86. Under English law the Directorswould be liable to the company, but not to any thirdparty, if the Report of the Directors contains errors asa result of recklessness or knowing misstatement ordishonest concealment of a material fact, but would nototherwise be liable.Report of the DirectorsPages 1-136 and 239-244 inclusive comprise the Reportof the Directors that has been drawn up and presentedin accordance with and in reliance upon English companylaw and the liabilities of the Directors in connectionwith that report shall be subject to the limitations andrestrictions provided by such law.WebsiteGlaxoSmithKline’s website www.gsk.com givesadditional information on the Group. Notwithstandingthe references we make in this Annual Report toGlaxoSmithKline’s website, none of the informationmade available on the website constitutes part of thisAnnual Report or shall be deemed to be incorporatedby reference herein.Alex SzabzonFront cover imageA child being seen by a doctor working for Brazil’sunified health system, which provides 95% ofscheduled vaccinations in the country. We have along-standing relationship with the governmentfunded science institution, Oswaldo Cruz Foundation,to manufacture vaccines for public health prioritiesin Brazil. This began with our first alliance in the1980s on polio vaccines and continues through torecent technology transfers for products like ourpneumococcal vaccine. Brazil is one of a number oflarge emerging market countries in which we arepresent and more than a quarter of the Group’s totalrevenues are now generated in these countries.Cautionary statement regardingforward-looking statementsThe Group’s reports filed with or furnished to the USSecurities and Exchange Commission (SEC), includingthis document and written information released, ororal statements made, to the public in the future by oron behalf of the Group, may contain forward-lookingstatements. Forward-looking statements give the Group’scurrent expectations or forecasts of future events. Aninvestor can identify these statements by the fact thatthey do not relate strictly to historical or current facts.They use words such as ‘anticipate’, ‘estimate’, ‘expect’,‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’ and other wordsand terms of similar meaning in connection with anydiscussion of future operating or financial performance.In particular, these include statements relating to futureactions, prospective products or product approvals,future performance or results of current and anticipatedproducts, sales efforts, expenses, the outcome ofcontingencies such as legal proceedings, and financialresults. The Group undertakes no obligation to updateany forward-looking statements, whether as a result ofnew information, future events or otherwise.Forward-looking statements involve inherent risksand uncertainties. The Group cautions investors thata number of important factors, including those in thisdocument, could cause actual results to differ materiallyfrom those contained in any forward-looking statement.Such factors include, but are not limited to, thosediscussed under ‘Risk factors’ on pages 78-86 of thisAnnual Report.A number of adjusted measures are used to report theperformance of our business. These measures are definedon page 56.Brand namesBrand names appearing in italics throughout this reportare trademarks either owned by and/or licensed toGlaxoSmithKline or associated companies, with theexception of Boniva/Bonviva, a trademark of Roche,NicoDerm, a trademark of Johnson & Johnson, Merrell,Novartis, Sanofi or GlaxoSmithKline, Potiga, a trademarkof Valeant, Prolia and Xgeva, trademarks of Amgen,Vesicare, a trademark of Astellas Pharmaceuticals inmany countries and of Yamanouchi Pharmaceuticals incertain countries, Volibris, a trademark of Gilead, Xyzal,a trademark of UCB or GlaxoSmithKline and Zyrtec, atrademark of UCB or GlaxoSmithKline all of which areused in certain countries under licence by the Group.
GSK Annual Report 20121OverviewFinancial review & riskFinancial review Financial position and resources Financial review 2011 Risk factors 55667278Governance & remunerationOur Board Our Corporate Executive Team Chairman’s letter Board report to shareholders Committee reports Remuneration CommitteeChairman’s letter Total remuneration for 2012 Pay for performance for 2012 Remuneration policy for 2013 Directors’ emolumentsand total remuneration Directors and Senior Management 109110111113127136138139140144218137-223 Financial statementsFinancial statementsDirectors’ statement ofresponsibilities Independent Auditors’ report Financial statements Notes to the financial statements Financial statements ofGlaxoSmithKline plc preparedunder UK GAAP 8892949510387-136 Governance & remunerationRead more at www.gsk.com2356812164955-86 Financial review & riskAs a global healthcare company, ourcommercial success depends on uscreating innovative new medicines,vaccines and healthcare products andmaking these accessible to as manypeople who need them as possible.2012 was characterised by achallenging global economic climate.Despite this, we have continued tomake good progress in our strategyto grow our business in a sustainableway, deliver new medicines andhealthcare products that are valuedby those who use them, and simplifyour operations.All of this has allowed us to deliversignificant returns to our shareholders.Strategic reviewChairman’s statement CEO’s review Strategic review How we performed What we do, Where we do it Our market How we deliver Responsible business 2-54 Strategic reviewGSK in 2012HighlightsTotal Group turnoverCore* operatingprofitTotal operatingprofit 6.3bn112.7p92.9p61stReturned toshareholdersKey medicines submittedfor regulatory approvalCore* earningsper shareTotal earningsper sharein Access toMedicines Index* The calculation of core results is described on page 56 and a reconciliation is provided on page 62.Investor informationProduct development pipeline 225Products, competition andintellectual property 229Quarterly trend 232Five year record 236Share capital and share price 239Dividends 240Annual General Meeting 2013 241US law and regulation 242Tax information for shareholders 243Analysis of shareholdings 244Shareholder services and contacts 245Glossary of terms and index 247224-248 Investor information 26.4bn 8.3bn 7.4bn
2 GSK Annual Report 2012Chairman’s statementDespite a challenging environment, I believe 2012 marked anotheryear of progress for GSK in the delivery of our strategy and ingenerating more sustainable returns to shareholdersOver the past five years, underSir Andrew’s leadership, the Group hasbeen fundamentally changing to improvegrowth prospects, reduce risk and deliverenhanced returns to shareholders.The benefits of this strategy wereevident during the year, with strongperformances in our emerging markets,and other growth businesses offsettingmuch of the impact of the significantlyworsening outlook in Europe. At the sametime, GSK’s R&D organisation deliveredunparalleled output with six key newproducts submitted for approval and thereis growing evidence that we can replenishthe late stage pipeline on a sustainablebasis. This is clearly of critical importanceto the longer-term prospects of the Group.Ultimately the aim of our strategy is todeliver sustainable earnings per sharegrowth (EPS) and improved returns toshareholders. GSK delivered flat core EPSof 112.7p but returned 6.3 billion toshareholders via dividends and buybacksin 2012. This brings to nearly 25 billionthe amount returned to shareholderssince Sir Andrew joined the Board atthe start of 2008.Operating in a responsible and ethicalway is essential for the commercialsuccess of GSK. As Chairman of theCorporate Responsibility Committee, Iwas pleased to see the continued progressduring the year in our efforts to improveglobal access to our medicines, withfurther agreements reached to supply ourvaccines to the world’s poorest countriesat low prices and to encourage researchinto neglected diseases. The Group alsotook industry-leading steps to improvetransparency of its clinical trial research.Oversight and management of riskremains a key focus for the Board.In July 2012, the Group successfullyresolved a series of long-standing legalmatters with the US Government. Theseprimarily related to historical sales andmarketing practices. The Board recognisesthat these matters do not reflect thecompany that GSK is today. Fundamentalchanges have been made to compliance,marketing and selling procedures inrecent years and significant progressmade to embed a culture in the companythat puts patients first and demandsintegrity in all behaviours and activities.We continue to make changes tothe Board as we plan for the futureand implement proactive successionplanning. I would like to thank bothSir Crispin Davis, who is standing downat this year’s AGM, and Larry Culp, whoretired from the Board in September, fortheir outstanding contributions overrecent years.In April, we appointed Lynn Elsenhansand Jing Ulrich as Non-ExecutiveDirectors. Respectively Lynn and Jinghave brought experience running globalcompanies and deep knowledge ofemerging markets to Board discussions.Additionally in January, we announcedthat Hans Wijers, currently chairmandesignate of Heineken and previouslyCEO of Akzo Nobel, will join GSK asa Non-Executive Director from thisyear’s AGM.I would also like to thank Sir RobertWilson for agreeing to remain on theBoard for an additional year to providecontinuity and advice as new Boardmembers settle into their roles.We have now met our original aspirationto have more than 25% femalerepresentation on the Board by 2013,and we remain committed to continuingto improve geographic and genderdiversity at Board level.In summary, while our operatingenvironment remains challenging,it is also not without substantialopportunity for companies that deliverinnovation and act with responsibility.The Board has every confidence in thestrength and resilience of Sir Andrewand his senior management team, andbelieves the Group is taking all thenecessary steps to build a stronger GSKthat can generate sustainable value forshareholders and society.Sir Christopher GentChairman
GSK Annual Report 2012We have diversified our sources of growth, our R&D productivityhas significantly improved and our processes are simpler and moreefficient. We are confident that our strategy is deliveringWe also made outstanding progress inresearch and development during theyear to advance potential new medicinesacross multiple disease areas includingrespiratory, oncology, diabetes and HIV.Investment in growth marketsIn emerging markets, the benefits ofinvestments made to increase ourexposure in Pharmaceuticals andVaccines, as well as Consumer Healthcare,were very evident. Total sales in emergingmarkets now account for 26% of ourbusiness and grew 10% during theyear. At a divisional level, ConsumerHealthcare sales were flat, but grew 5%,excluding divested OTC products.We are also confident that we cansustain this level of productivity andthat we can deliver our long-term goalof improving returns on R&D investmentto around 14%.Simplifying and changingour businessWe continue to make changes to simplifyour operating model. Our OperationalExcellence programme has now deliveredannual savings of 2.5 billion andremains on track to hit the target we setof 2.8 billion of annual savings by 2014.In February 2013 we announced a newmajor change programme, which weexpect to produce incremental annualcost savings of at least 1 billion by 2016.This programme will include a series oftechnological advances and opportunitiesto eliminate complexity, which webelieve can transform our long-term costcompetitiveness in both manufacturingand R&D. The programme will help ussimplify our supply chain processes,shorten cycle times, lower inventorylevels and reduce our carbon footprint.224-248 Investor informationIn Pharmaceutical and Vaccines, Japan’ssales fell 6%, reflecting the impact of theCervarix vaccine sales for the catch upprogramme in the prior year. ExcludingCervarix, sales grew 5%. Sales in the USAwere down 2%; this was an improvementover 2011 when sales declined 5%. Wehave been re-shaping our US business toreflect changing market dynamics and toprepare for the launch of multiple newproducts. We continue to view the USAand Japan very positively, as marketsthat reward and are willing to pay forhealthcare innovation.In R&D, the Group made significantprogress in 2012. We now have six keynew products under regulatory reviewand expect Phase III data on 14 assetsin 2013 and 2014. In total, over the nextthree years, GSK has the potential tolaunch around 15 new medicines andvaccines globally.137-223 Financial statementsAlthough reported sales for the yearwere down 1% (CER), sales were flatadjusting for the disposal of our noncore Consumer Healthcare brands. Thisreflects continued strong performancefrom our ‘growth’ businesses, helping tooffset pressure in Western markets.R&D productivity providesplatform for growth87-136 Governance & remunerationHowever, there is no doubt that weare operating in a very challengingenvironment and in 2012 this wasparticularly evident in Europe. Despitethis, we were able to maintain coreearnings per share (CER), generate netcash inflows from operating activities of 7 billion (before legal settlements) andreturn over 6.3 billion to shareholders.The clear adverse impact to ourperformance in 2012 was weaker thanexpected sales from our Europeanbusiness, down 7%. Here, governmentausterity measures adversely impactedgrowth by approximately 6 percentagepoints during the year.55-86 Financial review & riskFive years ago we set out a strategy tore-shape GSK to increase growth, reducerisk and improve our long-term financialperformance. We have made goodprogress and 2012 provided furtherevidence of this.2-54 Strategic reviewCEO’s review3
4 GSK Annual Report 2012In addition, given the sustained shiftwe have witnessed in the Europeanreimbursement and pricing environment,we plan to initiate further restructuringof our European pharmaceuticals businessto reduce costs, improve efficiencies andreallocate resources to support identifiedgrowth opportunities in these markets.We are also evaluating further strategicoptions to ensure we are able to maximisethe value of our current and futureportfolio in Europe.This additional restructuring supportsour strategy to change the shape of ourbusiness and deliver sustainable longterm growth. In the short term, it willalso help to offset some of the pressurewe are seeing on our margin structureresulting from changes in our businessmix. We remain confident that as ourpipeline begins to contribute from theend of 2013, we can drive improvementin the core operating margin over themedium term.Strengthening our corebusinessOur Consumer Healthcare businesscontinues to make excellent progress aswe increase focus around a core portfolioof healthcare brands and emergingmarkets, where we are seeing verypositive consumption trends and benefitfrom sales and distribution synergieswith pharmaceuticals.Investments to maximise returns in thesemarkets continue. Last year, we openeda new innovation centre in China andhave now increased our shareholdingin our Indian subsidiary. In line withthis strategic focus, we have decided toinitiate a review evaluating all strategicoptions for the Lucozade and Ribenadrinks brands, which are primarilymarketed in established Western markets.These brands are iconic and the reviewwill look at the best ways to ensure theircontinued growth.Outside Consumer Healthcare, wecontinue to strengthen our core businessthrough acquisitions and equityinvestments. In 2012 we completedthree significant transactions withHuman Genome Sciences, Shionogi andTheravance to increase our share of theeconomics on key future growth assets.At the same time, we delivered targeteddivestments at the periphery of theGroup to realise value for shareholders,divesting Vesicare, multiple non-core OTCbrands and Australian pharmaceutical‘tail’ products.Operating with responsibilityWe remain committed to operatingresponsibly and during the year we madefurther advances on our agenda to ensureour behaviour and actions meet or exceedthe expectations of society.For example, we have taken several stepsto increase transparency of our clinicalresearch. We already publish all our trialresults whether positive or negative.We have now committed to go furtherand enable independent researchers toaccess the very detailed data that liesbehind these results. By being moreopen, we hope to help further scientificunderstanding and research.We also continue to expand access toour medicines to people living in thepoorest countries in the world. In 2012GSK was again ranked number 1 in theAccess to Medicines (ATM) Index whichassesses healthcare companies’ activitiesin this field. In addition, we expandedour efforts to tackle neglected tropicaldiseases and supply low-price vaccines tothe GAVI alliance for use in the world’spoorest countries. We also receivedfurther data on our candidate malariavaccine. While additional analysis isneeded, this vaccine continues to havethe potential to save the lives of hundredsof thousands of children in Africa.As the Chairman notes in his review,in July we also settled multipleinvestigations with the US Governmentand states, primarily relating to historicalsales and marketing practices. Thesematters originated in a different erafor the company, but we continue totake action at all levels to improve ourprocedures for compliance, marketingand selling and embed a values-basedculture in GSK.OutlookGSK’s globally diversified sales base andimproved R&D output provide a clearplatform for growth, with 2013 markingthe start of what should be a series ofgrowth years for the Group.Specifically we expect to deliver core EPSgrowth of 3-4% CER and sales growthof around 1% CER during the year.*We also expect to deliver further strongcash generation in 2013 and remaincommitted to using free cash flow tosupport increasing dividends, sharerepurchases or, where returns are moreattractive, bolt-on acquisitions.In closing, I would like to thank allour employees, partners and suppliersfor their continued commitment andsupport. We are more confident thanever that GSK is well placed to succeedin emerging and pro-innovation marketsand that our R&D model is working.This is creating clear, long-term capacityfor GSK to deliver continued innovationand benefit to patients, and sustainedperformance and returns to shareholders.Sir Andrew WittyChief Executive Officer* All forward looking statements are based on 2012 restated numbers adjusted for IAS 19R (EPS of 111.4p), at CER and barring unforeseencircumstances. See ‘Cautionary statement regarding forward-looking statements’ on the inside front cover and page 56 for an explanation of CER.
GSK Annual Report 2012Ulverston in the Lake District in the north of Englandwill be the location for our new biopharmaceuticalmanufacturing centre – the first new factory GSKhas built in the UK for almost 40 years. This formspart of a series of UK investments of more than 500 million, made possible by the introductionof new patent box rules in the UK.55-86 Financial review & riskCapital investment68101216183042492-54 Strategic reviewStrategicreviewHow we performed What we do, Where we do it How we create value Our market How we deliver Grow a diversified global business Deliver more products of value Simplify the operating model Responsible business 587-136 Governance & remuneration137-223 Financial statementsTom Whipps224-248 Investor information
6 GSK Annual Report 2012Strategic reviewHow we performedWe measure our performance against a number of key indicators,and use core results for our planning and reporting purposesGroup turnoverFree cash flow b,c 26.4bn 2.0bn(1)(3)(1)Reported growth CER %(15)(8)(51)Reported growth %–(4)(3)Reported growth %19(14)(17)Growth excluding legal settlements %28.427.426.4How we performedReported sales were down 1% but were flatadjusting for the disposal of our non-core OTCConsumer Healthcare brands. Overall, strongperformances in EMAP and other growthbusinesses largely offset declines in USAand Europe.4.5How we performedFree cash flow was 2.0 billion. Excludinglegal settlements, adjusted free cash flow was 4.7 billion.4.12.0Why it’s importantA key objective of our strategy is to deliversustainable, broadly-sourced sales growth.2010201120122010Why it’s importantThis measure shows the cash we generatethat is available to return to shareholdersor reinvest in the business, as well as oureffectiveness in converting our earnings tocash through effective working capital controland investment discipline.20122011Core operating profit and marginaTotal operating profit and margin 8.3bn 7.4bn(4)(6)(3)Reported growth CER %(59) 100(3)Reported growth CER %–(7)(5)Reported growth %(55) 100(5)Reported growth %32.1%31.5%33.4%9.58.88.3How we performedCore operating profit was 8.3 billion. Coreoperating margin declined 0.6 percentagepoints to 31.5%, of which 0.3 percentagepoints was due to the expected impact ofthe acquisition of Human Genome Sciences.Why it’s importantOur objective remains to improve operatingleverage. The margin indicates how costs arebeing managed as sales 7.8How we performedTotal operating profit was 7.4 billion. Totaloperating margin declined 0.5 percentagepoints to 28.0%, of which 0.3 percentagepoints was due to the expected impact ofthe acquisition of Human Genome Sciences.Core earnings per shareaTotal earnings per eported growth CER %(75) 100(9)Reported growth CER %Reported growth %(71) 100(11)Reported growth %How we performedEffective cost control and delivery of financialefficiencies enabled the Group to deliver coreEPS of 112.7pWhy it’s importantEPS shows the portion of our profit allocatedto each share. It is a key indicator of ourperformance and the returns we are w we performedNon-core items included a tax charge of 420 million (8.6p) arising from thecentralisation of Pharmaceutical intellectualproperty and product inventory ownershipin the UK. Transactions completed in 2012resulted in a number of significant non-cashaccounting entries. However, these largelyoffset each other.
GSK Annual Report 201272-54 Strategic reviewNew Pharmaceuticals and Vaccines product performanceb 14.3bn 1.4bn5114.65314.45414.3% share of total turnoverHow we performedWe saw continued growth in emerging markets,Japan (excluding vaccines) and ConsumerHealthcare (excluding disposals). Performancein Vaccines was impacted by reduced sales ofCervarix following the HPV vaccinationcatch-up programme in Japan in 2011.36471.71.420122010Cash returned to shareholders 10201120122012Relative total shareholder returnb,d160Reported growth %How we performedDuring 2012, GSK returned 6.3 billion toshareholders via dividends and sharebuy-backs.Why it’s importantWe continue to focus on delivering dividendgrowth and returning free cash flow toshareholders through share buy-backs wherethis offers a more attractive return thanalternative investments.a We use a number of adjusted measures to report the SmithKline Total ReturnFTSE 100 Total Return Index31/12/1031/12/1131/12/12GlaxoSmithKline Pharma Peers Return Indexb The remuneration of our executives is linked to the marked keyindicators. Further information on our executive pay policy can befound in our Remuneration report on page 109.c The calculation of free cash flow is described on page 56 and areconciliation is provided on page 69. The calculation of CER isdescribed on page 56.d The constituents of the Pharma Peers Return Index are listed onpage 115.224-248 Investor informationof our business. These include core results, which are used bymanagement for planning and reporting purposes and may notbe directly comparable with similarly described measures used byother companies. Core results exclude a number of items from totalresults. A full definition of core results can be found on page 56 anda reconciliation between core results and total results is providedon page 62.140137-223 Financial statements3.2Buybacks6.35.62.22011Why it’s importantThis measure shows the delivery of salesfrom products launched in the prior fiveyears and creates incentives for improvedR&D performance.87-136 Governance & remuneration2011Reported growth CER %How we performedTotal sales of new products were 1.4 billion,grew 34% in the year and represented 7%of Pharmaceutical and Vaccines turnover.2012 was impacted by the roll-off of productsmore than five years old.2.5Why it’s importantThis measure focuses on our major growthareas: Vaccines, Consumer Healthcare, EMAP,Japan and dermatology.20103455-86 Financial review & riskTurnover in our major growth areasb
8 GSK Annual Report 2012Strategic reviewWhat we doWe are a science-led global healthcare company that researchesand develops a broad range of innovative productsOur businessWe have three primary areas of business Pharmaceuticals,Vaccines and Consumer Healthcare. Our objective is to deliversustainable growth across this portfolio. 26.4bnPharmaceuticalsVaccinesConsumer Healthcare 18.0bn 68% 3.3bnOur Pharmaceuticals business developsand makes available medicines to treat abroad range of serious and chronic diseases.Our portfolio is made up of establishedbrands and newer innovative patentprotected medicines.Our Vaccines business is one of the largestin the world, producing paediatric andadult vaccines against a range of infectiousdiseases. In 2012, we distributed nearly 900million doses to 170 countries, of which over80% were supplied to developing countries.We develop and market a range ofconsumer health products based onscientific innovation. We have brandsin four main categories: Total wellness,Oral care, Nutrition and Skin health.Sales by therapy area Sales by vaccine Sales by category Turnoverof GroupTurnover mRespiratory 7,291Anti-virals 753Central nervous system 1,670Cardiovascular and urogenital 2,431Metabolic 171Anti-bacterials 1,247Oncology and emesis 798Dermatology 850495Rare diseases Immuno-inflammation 70ViiV Healthcare (HIV) 1,374Other 846Read more on page 57Group turnover13% 5.1bnof GroupTurnover mBoostrix 238Cervarix 270Fluarix, FluLaval 200646Hepatitis Infanrix, Pediarix 775Rotarix 360Synflorix 385Other 45119%of Group mTotal wellness 2,008Oral care 1,797Nutrition 1,050255Skin health Read more on page 58Read more on page 59R&DOur business is sustained through investment in R&D. In 2012we spent 3.5 billion before non-core items*, 4.0 billion in total,in our search to develop new medicines, vaccines and innovativeconsumer products.During the year we saw significant delivery from our late stagepipeline, with six key medicines filed with regulators.We have dedicated research programmes for diseases that affectthe developing world. We are one of the few healthcare companiesresearching both new vaccines and new medicines for all three ofthe World Health Organization’s priority diseases: HIV/AIDS, malariaand tuberculosis. 3.5bnCore R&Dexpenditurein 2012c.30Assets in latestage pipelineCore R&D expenditure allocation in 2012PharmaceuticalsVaccinesConsumer HealthcareRead more on page 32* The calculation of core results and non-core items is set out on page 56. m%2,82149815581145
GSK Annual Report 20122-54 Strategic reviewWhere we do itOur geographic presence covers more than 100 countries55-86 Financial review & riskOur global reachThe shape of our business is shifting to capitalise on marketswith high-growth potential including those in Asia Pacific,Latin America and Japan. Territories outside the USA andEurope now account for 40% of our total sales.99,488EmployeesEmployees by regionWe have a significant global manufacturing and R&D presencewith a network of 87 manufacturing sites and large R&D centresin the UK, USA, Spain, Belgium and China.94 5 383,5153,246Turnover by region514 12345 37-223 Financial statements2387-136 Governance & remunerationHow we’re structuredTurnover by segmentFor Pharmaceuticals and Vaccines, we operate in geographicalsegments that combine these two businesses. Our ConsumerHealthcare business functions as a global unit, as does ViiV Healthcare,the specialist HIV company we founded with Pfizer in 2009.US Pharmaceuticals and VaccinesEurope Pharmaceuticals and VaccinesEMAP Pharmaceuticals and VaccinesJapan Pharmaceuticals and VaccinesViiV HealthcareOther tradingConsumer HealthcareOther trading turnover includes Canada, Puerto Rico, Australasia,central vaccine tender sales and contract manufacturing sales.Read more on page 151 bn7.05.04.72.01.41.25.1224-248 Investor informationOur commercial businesses are structured around regional unitsor areas of focus.
10 GSK Annual Report 2012Strategic reviewHow we create valueBy delivering innovation and expanding access to ourproducts we create value for society and our shareholdersThe contextWe see both opportunities and challenges inour operating environment. Scientific researchis continuously uncovering new understandings
people to do more, feel better and live longer. Our mission Notice regarding limitations on Director Liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Report of the Directors conta