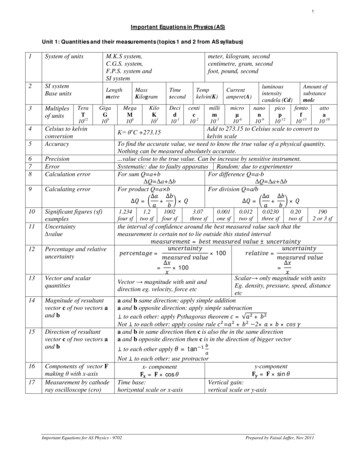
Transcription
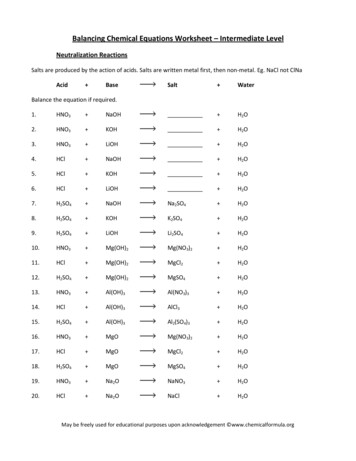
Balancing Chemical Equations Worksheet – Intermediate LevelNeutralization ReactionsSalts are produced by the action of acids. Salts are written metal first, then non-metal. Eg. NaCl not ClNaAcid BaseSalt WaterBalance the equation if required.1.HNO3 NaOH H2O2.HNO3 KOH H2O3.HNO3 LiOH H2O4.HCl NaOH H2O5.HCl KOH H2O6.HCl LiOH H2O7.H2SO4 NaOHNa2SO4 H2O8.H2SO4 KOHK2SO4 H2O9.H2SO4 LiOHLi2SO4 H2O10.HNO3 Mg(OH)2Mg(NO3)2 H2O11.HCl Mg(OH)2MgCl2 H2O12.H2SO4 Mg(OH)2MgSO4 H2O13.HNO3 Al(OH)3Al(NO3)3 H2O14.HCl Al(OH)3AlCl3 H2O15.H2SO4 Al(OH)3Al2(SO4)3 H2O16.HNO3 MgOMg(NO3)2 H2O17.HCl MgOMgCl2 H2O18.H2SO4 MgOMgSO4 H2O19.HNO3 Na2ONaNO3 H2O20.HCl Na2ONaCl H2OMay be freely used for educational purposes upon acknowledgement www.chemicalformula.org
21.H2SO4 Na2ONa2SO4 H2O22.HNO3 K2OKNO3 H2O23.HCl K2OKCl H2O24.H2SO4 K2OK2SO4 H2O25.HNO3 CaOCa(NO3)2 H2O26.HCl CaOCaCl2 H2O27.H2SO4 CaOCaSO4 H2O28.HNO3 Ba(OH)2Ba(NO3)2 H2O29.HCl Al2O3AlCl3 H2O30.H2SO4 Ba(OH)2BaSO4 H2OAcetate salts, are written acetate group first and metal second. The acetate group, CH3COO- is written first asthe negative charge is on the oxygen atom and not the carbon atom. The negative oxygen atom is attracted tothe positive metal ion. Eg. Sodium acetate, formula CH3COONa is really CH3COO-Na and not Na CH3COO31.CH3COOH NaOHCH3COONa H2O32.CH3COOH KOHCH3COOK H2O33.CH3COOH LiOHCH3COOLi H2O34.CH3COOH Na2OCH3COONa H2O35.CH3COOH K2OCH3COOK H2O36.CH3COOH CaO(CH3COO)2Ca H2OThe reaction of an acid with ammonia, NH3 does not produce water.37.HNO3 NH3NH4NO338.HCl NH339.H2SO4 NH3(NH4)2SO440.CH3COOH NH3CH3COONH4May be freely used for educational purposes upon acknowledgement www.chemicalformula.org
Balancing Chemical Equations Worksheet – Advanced LevelNeutralization ReactionsSalts are produced by the action of acids. Salts are written metal first, then non-metal. Eg. NaCl not ClNaAcid BaseSalt WaterIdentify the salt produced and balance the equation if required.1.HNO3 NaOH H2O2.HNO3 KOH H2O3.HNO3 LiOH H2O4.HCl NaOH H2O5.HCl KOH H2O6.HCl LiOH H2O7.H2SO4 NaOH H2O8.H2SO4 KOH H2O9.H2SO4 LiOH H2O10.HNO3 Mg(OH)2 H2O11.HCl Mg(OH)2 H2O12.H2SO4 Mg(OH)2 H2O13.HNO3 Al(OH)3 H2O14.HCl Al(OH)3 H2O15.H2SO4 Al(OH)3 H2O16.HNO3 MgO H2O17.HCl MgO H2O18.H2SO4 MgO H2O19.HNO3 Na2O H2O20.HCl Na2O H2OMay be freely used for educational purposes upon acknowledgement www.chemicalformula.org
21.H2SO4 Na2O H2O22.HNO3 K2O H2O23.HCl K2O H2O24.H2SO4 K2O H2O25.HNO3 CaO H2O26.HCl CaO H2O27.H2SO4 CaO H2O28.HNO3 Ba(OH)2 H2O29.HCl Al2O3 H2O30.H2SO4 Ba(OH)2 H2OAcetate salts, are written acetate group first and metal second. The acetate group, CH3COO- is written first asthe negative charge is on the oxygen atom and not the carbon atom. The negative oxygen atom is attracted tothe positive metal ion. Eg. Sodium acetate, formula CH3COONa is really CH3COO-Na and not Na CH3COO31.CH3COOH NaOH H2O32.CH3COOH KOH H2O33.CH3COOH LiOH H2O34.CH3COOH Na2O H2O35.CH3COOH K2O H2O36.CH3COOH CaO H2OThe reaction of an acid with ammonia, NH3 does not produce water.37.HNO3 NH338.HCl NH339.H2SO4 NH340.CH3COOH NH3May be freely used for educational purposes upon acknowledgement www.chemicalformula.org
Balancing Chemical Equations Worksheet - ANSWERS already balancedNeutralization ReactionsAcid BaseSalt Water1.HNO3 NaOHNaNO3 H2O 2.HNO3 KOHKNO3 H2O 3.HNO3 LiOHLiNO3 H2O 4.HCl NaOHNaC l H2O 5.HCl KOHKCl H2O 6.HCl LiOHLiCl H2O 7.H2SO4 2NaOHNa2SO4 2H2O8.H2SO4 2KOHK2SO4 2H2O9.H2SO4 2LiOHLi2SO4 2H2O10.2HNO3 Mg(OH)2Mg(NO3)2 2H2O11.2HCl Mg(OH)2MgCl2 2H2O12.H2SO4 Mg(OH)2MgSO4 2H2O13.3HNO3 Al(OH)3Al(NO3)3 3H2O14.3HCl Al(OH)3AlCl3 3H2O15.3H2SO4 2Al(OH)3Al2(SO4)3 6H2O16.2HNO3 MgOMg(NO3)2 H2O17.2HCl MgOMgCl2 H2O18.H2SO4 MgOMgSO4 H2O19.2HNO3 Na2O2NaNO3 H2O20.2HCl Na2O2NaCl H2O May be freely used for educational purposes upon acknowledgement www.chemicalformula.org
21.H2SO4 Na2ONa2SO4 H2O22.2HNO3 K2O2KNO3 H2O23.2HCl K2O2KCl H2O24.H2SO4 K2OK2SO4 H2O25.2HNO3 CaOCa(NO3)2 H2O26.2HCl CaOCaCl2 H2O27.H2SO4 CaOCaSO4 H2O28.2HNO3 Ba(OH)2Ba(NO3)2 2H2O29.6HCl Al2O32AlCl3 3H2O30.H2SO4 Ba(OH)2BaSO4 2H2O Acetate salts, are written acetate group first and metal second. The acetate group, CH3COO- is written first asthe negative charge is on the oxygen atom and not the carbon atom. The negative oxygen atom is attracted tothe positive metal ion. Eg. Sodium acetate, formula CH3COONa is really CH3COO-Na and not Na CH3COO31.CH3COOH NaOHCH3COONa H2O 32.CH3COOH KOHCH3COOK H2O 33.CH3COOH LiOHCH3COOLi H2O 34.2CH3COOH Na2O2CH3COONa H2O35.2CH3COOH K2O2CH3COOK H2O36.2CH3COOH CaO(CH3COO)2Ca H2OThe reaction of an acid with ammonia, NH3 does not produce water.37.HNO3 NH3NH4NO3 38.HCl NH3NH4Cl 39.H2SO4 2NH3(NH4)2SO440.CH3COOH NH3CH3COONH4 May be freely used for educational purposes upon acknowledgement www.chemicalformula.org
The reaction of an acid with ammonia, NH 3 does not produce water. 37. HNO 3 NH 3 NH 4 NO 3 38. HCl NH 3 _ 39. H 2 SO 4 NH 3 (NH 4) 2 SO 4 40. CH 3 COOH NH 3 CH 3 COONH 4. May be freely used for educational purposes upon acknowledgement www.chemicalformula.org Balancing Chemical Equations Worksheet – Advanced Level Neutralization Reactions Salts are produced by the