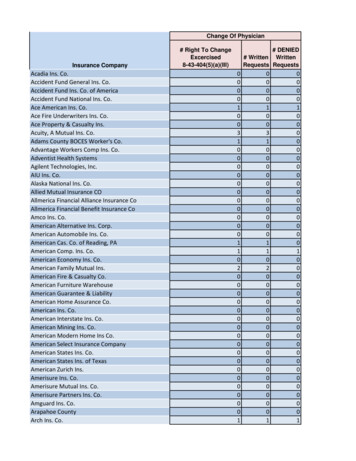
Transcription
Advanced Control Formulary Change Summary ReportEffective 04-01-2020This report highlights all changes (additions, deletions, and removals) to the CVS Caremark AdvancedControl yProductBrand Agents:Vascepa (icosapentethyl)oral tilipemics/ Omega-3Fatty AcidsVascepa is indicated as an adjunct to diet toreduce triglyceride (TG) levels in adultpatients with severe ( 500 mg/dL)hypertriglyceridemia.To provide an additional option for reducing triglyceridelevels in those with high triglycerides.buprenorphinetransdermaltransdermal systemAnalgesics/ OpioidAnalgesicsTo provide an additional generic option for severe painmanagement.fenofibric aciddelayed-reloral delayed-releasecapsuleCardiovascular/Antilipemics/ FibratesBuprenorphine is indicated for themanagement of pain severe enough torequire daily, around-the-clock, long-termopioid treatment and for which alternativetreatment options are inadequate.Fenofibric acid delayed-release capsule isindicated as adjunctive therapy to diet to: Reduce TG in patients with severehypertriglyceridemia Reduce elevated LDL-C, Total-C, TGand Apo B, and to increase HDL-C inpatients with primaryhypercholesterolemia or mixeddyslipidemiaGeneric Agents:To provide an additional generic option for reducing lipidlevels in those with high lipids.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 1 of 14
Advanced Control Formulary Change Summary ReportEffective ntanyltransmucosalbuccal tabletAnalgesics/ OpioidAnalgesicsfulvestrantintramuscular injectionAntineoplastic Agents/Hormonal AntineoplasticAgents/ AntiestrogensIndicationOptions/CommentsFentanyl transmucosal is indicated for themanagement of breakthrough pain incancer patients 18 years of age and olderwho are already receiving and who aretolerant to around-the-clock opioid therapyfor their underlying persistent cancer pain.Fulvestrant is indicated for the treatment of: Hormone receptor (HR)-positive, humanepidermal growth factor receptor 2(HER2)-negative advanced breastcancer in postmenopausal women notpreviously treated with endocrinetherapy HR-positive advanced breast cancer inpostmenopausal women with diseaseprogression following endocrine therapy HR-positive, HER2-negative advancedor metastatic breast cancer inpostmenopausal women in combinationwith ribociclib, as initial endocrine basedtherapy or following diseaseprogression on endocrine therapy HR-positive, HER2-negative advancedor metastatic breast cancer incombination with palbociclib orabemaciclib in women with diseaseprogression after endocrine therapyTo provide an additional generic option for breakthroughpain management in those with cancer.To provide an additional option for the treatment ofhormone receptor (HR)-positive, human epidermalgrowth factor receptor 2 (HER2)-negative breast cancer.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 2 of 14
Advanced Control Formulary Change Summary ReportEffective salamine delayedrel capsuleoral y BowelDisease/ Oral Agentspregabalinoral capsule, oralsolutionCentral NervousSystem/ Fibromyalgiaramelteonoral tabletCentral NervousSystem/ cstriamtereneoral capsuleIndicationOptions/CommentsMesalamine delayed-release capsule isindicated for: Treatment of mildly to moderately activeulcerative colitis in patients 5 years ofage and older Maintenance of remission of ulcerativecolitis in adultsPregabalin is indicated for: Neuropathic pain associated withdiabetic peripheral neuropathy (DPN) Postherpetic neuralgia (PHN) Adjunctive therapy for the treatment ofpartial-onset seizures in patients 1month of age and older Fibromyalgia Neuropathic pain associated with spinalcord injuryRamelteon is indicated for the treatment ofinsomnia characterized by difficulty withsleep onset.Triamterene is indicated in the treatment of: Edema associated with congestiveheart failure, cirrhosis of the liver andthe nephrotic syndrome Steroid-induced edema, idiopathicedema and edema due to secondaryhyperaldosteronismTo provide an additional generic option for the treatmentof ulcerative colitis.To provide a generic option for the management ofneuropathic pain associated with diabetic peripheralneuropathy or spinal cord injury, postherpetic neuralgia,partial-onset seizures, and fibromyalgia.To provide an additional generic option for the treatmentof insomnia.To provide an additional generic potassium-sparingdiuretic option.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 3 of 14
Advanced Control Formulary Change Summary ReportEffective ncomycin capsuleoral omycin is indicated in the treatment of: C. difficile-associated diarrhea Enterocolitis caused by Staphylococcusaureus (including methicillin-resistantstrains)Options/CommentsTo provide a generic oral option for the treatment of C.difficile-associated diarrhea and enterocolitis caused byStaphylococcus oductBrand Agents:Faslodex (fulvestrant)intramuscular injectionAntineoplastic Agents/Hormonal AntineoplasticAgents/ AntiestrogensIndicationOptions/CommentsFaslodex is indicated for the treatment of: Hormone receptor (HR)-positive, humanepidermal growth factor receptor 2(HER2)-negative advanced breastcancer in postmenopausal women notpreviously treated with endocrinetherapy HR-positive advanced breast cancer inpostmenopausal women with diseaseprogression following endocrine therapy HR-positive, HER2-negative advancedor metastatic breast cancer inpostmenopausal women in combinationwith ribociclib, as initial endocrine basedAvailability of a generic option for the treatment ofhormone receptor (HR)-positive, human epidermalgrowth factor receptor 2 (HER2)-negative breast cancer.The preferred option on the Advanced ControlFormulary is fulvestrant.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 4 of 14
Advanced Control Formulary Change Summary ReportEffective rica (pregabalin)oral capsule, oralsolutionCentral NervousSystem/ FibromyalgiaIndicationOptions/Commentstherapy or following diseaseprogression on endocrine therapy HR-positive, HER2-negative advancedor metastatic breast cancer incombination with palbociclib orabemaciclib in women with diseaseprogression after endocrine therapyLyrica is indicated for: Neuropathic pain associated withdiabetic peripheral neuropathy (DPN) Postherpetic neuralgia (PHN) Adjunctive therapy for the treatment ofpartial-onset seizures in patients 1month of age and older Fibromyalgia Neuropathic pain associated with spinalcord injuryAvailability of additional options for the management ofneuropathic pain associated with diabetic peripheralneuropathy or spinal cord injury, postherpetic neuralgia,partial-onset seizures, and fibromyalgia.Preferred options on the Advanced Control Formularyinclude carbamazepine, carbamazepine ext-rel,divalproex sodium, divalproex sodium ext-rel,gabapentin, lamotrigine, lamotrigine ext-rel,levetiracetam, levetiracetam ext-rel, oxcarbazepine,phenobarbital, phenytoin, phenytoin sodium extended,pregabalin, primidone, tiagabine, topiramate,valproic acid, zonisamide, Fycompa (perampanel),Gralise (gabapentin ext-rel), Oxtellar XR (oxcarbazepineext-rel), Trokendi XR (topiramate ext-rel), and Vimpat(lacosamide).This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 5 of 14
Advanced Control Formulary Change Summary ReportEffective ProductBrand Agents:Atopaderm(mechanical allergenparticle barrier)topical creamCicatrace (silicone gelmatrix)topical sheetTopical/ Dermatology/Wound Care ProductsTopical/ Dermatology/Scar TreatmentEnteraGam (serumderived bovineimmunoglobulin/proteinisolate, SBI)oral packetGastrointestinal/MiscellaneousNicadan plements/Vitamins and Minerals/Folic Acid/ CombinationsIndicationOptions/CommentsAtopaderm is indicated to: Manage and relieve the burning, itchingand pain experienced with various typesof dermatoses, including atopicdermatitis, allergic contact dermatitisand radiation dermatitis Relieve dry, waxy skin by maintaining amoist wound and skin environment,which is beneficial to the healingprocessCicatrace is indicated for non-invasivemanagement of old and new hypertrophic orkeloid scars resulting from burns, generalsurgical procedures, injuries or traumawounds.EnteraGam is a medical food productintended for the dietary management ofchronic diarrhea and loose stools.Availability of generic options to manage and relieve theburning, itching and pain experienced with various typesof dermatoses.Nicadan is a dietary supplement that isbeneficial in maintaining overall skin health,Preferred options on the Advanced Control Formularyinclude desonide and hydrocortisone.Availability of additional options for the management ofhypertrophic or keloid scars.Consult doctor for preferred options on the AdvancedControl Formulary.Availability of additional options for the management ofchronic diarrhea and loose stools.Preferred options on the Advanced Control Formularyinclude alosetron, Viberzi (eluxadoline), and Xifaxan 550mg (rifaximin).Availability of additional supplementation options.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 6 of 14
Advanced Control Formulary Change Summary ReportEffective drochloride-folicacid-magnesiumcitrate-zinc gluconatecopper gluconate-alphalipoic acid)oral tabletPolytoza (occlusivesilicone sheet)topical sheetTopical/ Dermatology/Scar cteriumanimalis lactis)oral tabletNutritional/Supplements/Vitamins and Minerals/MiscellaneousScarSilk Pad(occlusive siliconesheet)topical patchTopical/ Dermatology/Scar TreatmentSilivex(occlusive siliconesheet)topical sheetTopical/ Dermatology/Scar TreatmentIndicationOptions/Commentsespecially in patients with inflammatory skinconditions.Preferred options on the Advanced Control Formularyinclude folic acid and folic acid-vitamin B6-vitamin B12.Polytoza can be used for the managementof hypertrophic and keloid scars.Availability of additional options for the management ofhypertrophic or keloid scars.Prodigen is indicated for the clinical dietarymanagement of suboptimal nutritionalstatus in patients where advancedsupplementation is required and nutritionalsupplementation in physiologically stressfulconditions for maintenance of good health isneeded.ScarSilk Pad is indicated for managementof closed hyperproliferative scars, both oldand new hypertrophic or keloid scarsresulting from burns, surgical procedures ortrauma wounds.Silivex is indicated for management of bothexisting and new hypertrophic or keloidscars and as a prophylactic treatment onclosed wounds to prevent hypertrophic orConsult doctor for preferred options on the AdvancedControl Formulary.Availability of additional supplementation options.Consult doctor for preferred options on the AdvancedControl Formulary.Availability of additional options for the management ofhypertrophic or keloid scars.Consult doctor for preferred options on the AdvancedControl Formulary.Availability of additional options for the management ofhypertrophic or keloid scars.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 7 of 14
Advanced Control Formulary Change Summary ReportEffective ltrex (occlusivesilicone sheet)topical sheetTopical/ Dermatology/Scar TreatmentIndicationOptions/Commentskeloid scarring that may have resulted fromburns, surgical procedures or traumawounds.Siltrex is indicated for management, control,and prevention of old and new hypertrophicor keloid scars resulting from burns orsurgical or traumatic injury of the skin.Consult doctor for preferred options on the AdvancedControl Formulary.Activite is a dietary management productthat can be used where Vitamin B, VitaminC, and folic acid supplementation isnecessary.Acyclovir cream is indicated for thetreatment of recurrent herpes labialis (coldsores) in immunocompetent adults andadolescents 12 years of age and older.Availability of additional supplementation options.Availability of additional options for the management ofhypertrophic or keloid scars.Consult doctor for preferred options on the AdvancedControl Formulary.Generic Agents:Activite (B-complexvitamin C-folic acid)oral tabletNutritional/Supplements/Vitamins and Minerals/Folic Acid/ Combinationsacyclovir creamtopical creamTopical/ Dermatology/Herpes Agentschlordiazepoxideclidinium (NDC42494040901 only)oral xide-clidinium is indicated: To control emotional and somaticfactors in gastrointestinal disorders As adjunctive therapy in the treatmentof peptic ulcer and in the treatment ofthe irritable bowel syndrome and acuteenterocolitis.Preferred options on the Advanced Control Formularyinclude folic acid and folic acid-vitamin B6-vitamin B12.Availability of generic options for the treatment of coldsores.Preferred options on the Advanced Control Formularyinclude acyclovir (except acyclovir cream) andvalacyclovir.Availability of generic antispasmodic options for variousgastrointestinal disorders.Preferred options on the Advanced Control Formularyinclude dicyclomine, hyoscyamine sulfate, hyoscyaminesulfate ext-rel, and hyoscyamine sulfate orallydisintegrating tablet.This document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 8 of 14
Advanced Control Formulary Change Summary ReportEffective clobenzaprinetablet 7.5 mgoral tabletdexchlorpheniramineoral syrupFexmid(cyclobenzaprine tablet7.5 mg)oral tabletCentral NervousSystem/ MusculoskeletalTherapy AgentsRespiratory/AntihistaminesCentral NervousSystem/ MusculoskeletalTherapy AgentsIndicationCyclobenzaprine tablet 7.5 mg is indicatedas an adjunct to rest and physical therapyfor relief of muscle spasm associated withacute, painful musculoskeletal conditions.Dexchlorpheniramine is indicated for: Perennial and seasonal allergic rhinitis Vasomotor rhinitis Allergic conjunctivitis due to inhalantallergens and foods Mild, uncomplicated allergic skinmanifestations of urticaria andangioedema Amelioration of allergic reactions toblood or plasma Dermographism As therapy for anaphylactic reactionsadjunctive to epinephrine and otherstandard measures after the acutemanifestations have been controlledFexmid is indicated as an adjunct to restand physical therapy for relief of musclespasm associated with acute, painfulmusculoskeletal conditions.Options/CommentsAvailability of generic options for the management ofmuscle spasms.Preferred options on the Advanced Control Formularyinclude carisoprodol, chlorzoxazone, cyclobenzaprine(except 7.5 mg tablet), metaxalone, methocarbamol,and orphenadrine-aspirin-caffeine.Availability of generic antihistamine options.Preferred options on the Advanced Control Formularyinclude clemastine 2.68 mg, cyproheptadine, andlevocetirizine.Availability of generic options for the management ofmuscle spasms.Preferred options on the Advanced Control Formularyinclude carisoprodol, chlorzoxazone, cyclobenzaprineThis document contains references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceuticalmanufacturers not affiliated with CVS Caremark. The information contained in this document is proprietary. The information may not be copied inwhole or in part without written permission. 2020 CVS Caremark. All rights reserved.106-40278A 013120Pg. 9 of 14
Advanced Control Formulary Change Summary ReportEffective lvite-D (folic acidcholecalciferol)oral tabletNutritional/Supplements/Vitamins and Minerals/Folic Acid/ Combinationsketoconazole foam2%topical foamTopical/ Dermatology/AntifungalsKetodan Foam(ketoconazole)topical foamketoprofen ext-relcapsuleoral extended-releasecapsuleTopical/ Dermatology/AntifungalsAnalgesics/ NSAIDsIndicationFolvite-D is indicated for dietarymanagement of patients with uniquenutritional needs requiring increased folatelevels, Vitamin D levels, or used inimproving the nutritional status of patientswith folic acid and vitamin D deficiency.Ketoconazole foam is indicated for topicaltreatment of seborrheic dermatitis inimmunocompetent patients 12 years of ageand older.Ketodan Foam is indicated for topicaltreatment of seborrheic dermatitis inimmunocompetent patients 12 years of ageand older.Ketoprofen extended-release capsules areindicated for the management of the signsand symptoms of rheumatoid arthritis andosteoarthritis.Options/Comments(except 7.5 mg tablet), metaxalone, methocarbamol,and orphenadrine-aspirin-caffeine.Availability of additional supplementation options.Preferred options on the Advanced Control Formularyinclude folic acid and folic acid-vitamin B6-vitamin B12.Availability of other topical options for the treatment ofseborrheic dermatitis.Preferred options on the Advanced Control Formularyinclude ketoconazole shampoo 2% and selenium sul
ulcerative colitis in patients 5 years of age and older Maintenance of remission of ulcerative colitis in adults To provide an additional generic option for the treatment . EnteraGam is a medical food product intended for the