Transcription
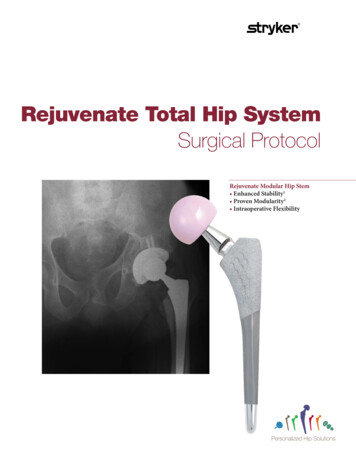
OrthopaedicsRejuvenate Modular Hip SystemOutcomes StudyCLINICAL PROTOCOLA prospective, post-market, multi-center study of the outcomes of theRejuvenate Modular Hip SystemSponsor:Stryker Orthopaedics325 Corporate DriveMahwah, NJ 07430Phone: 201-831-5000Clinical Study Manager:Christina Hawley325 Corporate DriveMahwah, NJ 07430Phone: 858-344-9792Study Product:Rejuvenate Modular Hip SystemProtocol Number:68IDE Number:N/AVersion 1.0Date: 10/7/2009CONFIDENTIALThis document is confidential and the property of Stryker Orthopaedics. No partof it may be transmitted, reproduced, published or used by other persons withoutprior written authorization from Stryker.
OrthopaedicsProtocol Change HistoryVersion1.010/7/2009DescriptionChanged ByNewDiana F. DoumatoCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page iiTable of ContentsSTUDY SYNOPSIS . 1EVALUATION SCHEDULE . 61INTRODUCTION. 71.11.21.31.42BACKGROUND . 7INVESTIGATIONAL DEVICE. 9PRECLINICAL DATA . 10CLINICAL DATA TO DATE . 10STUDY OBJECTIVES . 102.1EFFICACY . 102.1.1 Primary . 102.1.2 Secondary . 112.2SAFETY . 153CLINICAL STUDY PLAN . 163.13.23.33.44ELIGIBILITY . 174.14.25STUDY DEVICE . 18DEVICE RETRIEVAL PROCESS . 24EVALUATIONS . 257.17.27.37.48TREATMENT ASSIGNMENT . 18RANDOMIZATION . 18DEVICE DESCRIPTION . 186.16.27INCLUSION CRITERIA . 17EXCLUSION CRITERIA . 18SUBJECT ENROLLMENT . 185.15.26STUDY DESIGN . 16NUMBER OF CENTERS . 16NUMBER OF SUBJECTS. 16ESTIMATED STUDY DURATION . 16PREOPERATIVE VISIT . 25SURGERY . 266-WEEK VISIT . 27ANNUAL FOLLOW-UP VISITS . 28ADVERSE EVENTS . 298.1REPORTING OF ADVERSE EVENTS . 298.2GENERAL ADVERSE EVENT DEFINITIONS . 318.3STUDY SPONSOR FAX NOTIFICATION BY INVESTIGATOR . 338.3.1 Ethics Committee/Institutional Review Board Notification by Investigator . 338.4RECORDING OF ADVERSE EVENTS. 348.5MEDICAL MONITORING . 349STATISTICAL PLAN . 34CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page iii9.1EFFICACY . 349.1.1 Primary Efficacy Parameters . 349.1.2 Secondary Efficacy Parameters . 349.1.3 Primary Efficacy Hypothesis . 349.1.4 Primary Efficacy Analysis . 359.1.5 Secondary Efficacy Analysis . 359.2SAFETY PARAMETERS . 359.2.1 Safety Parameters. 359.2.2 Safety Analysis . 359.3MISSING DATA . 369.4STATISTICAL METHODOLOGY . 369.4.1 Data Summary . 369.4.2 Sample Size Calculation . 369.4.3 Interim Analyses and early Stopping Considerations . 379.4.4 Patient Populations . 3710STUDY PROCEDURES. 3710.110.210.311DATA MANAGEMENT . 4011.111.211.311.411.511.611.712DATABASE . 40CONFIDENTIALITY . 40SOURCE DOCUMENTS . 41CASE REPORT FORMS . 41DATA CLARIFICATION REQUESTS . 42PROTOCOL DEVIATIONS . 42RECORDS RETENTION . 42RISK/BENEFIT ASSESSMENT . 4312.112.212.312.412.513SUBJECT RECRUITMENT AND SCREENING . 37PATIENT INFORMED CONSENT AND GUIDELINES . 38EARLY WITHDRAWAL OF SUBJECTS . 39RISK CATEGORY . 43POTENTIAL RISK . 43EXPECTED COMPLICATIONS AND RATES OF OCCURRENCES . 44PROTECTION AGAINST RISKS . 46POTENTIAL BENEFITS TO THE SUBJECT . 46STUDY MONITORING, AUDITING, AND INSPECTING . 4713.113.2STUDY MONITORING PLAN . 47AUDITING AND INSPECTING . 4714ETHICAL CONSIDERATIONS . 4815STUDY FINANCES. 4815.115.215.3FUNDING SOURCE . 48CONFLICT OF INTEREST . 48SUBJECT STIPENDS OR PAYMENTS . 4916PUBLICATION PLAN . 4917REFERENCES. 51CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page ivCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page vList of AppendicesAppendix ASuggested Radiographic TechniqueAppendix BBiomechanical MeasurementsAppendix CComponent ListAppendix DStudy AdvertisementsAppendix EModel Informed Patient ConsentAppendix FDraft Case Report FormsAppendix GPatient Retention ProgramCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page viList of TablesTable 1. Rejuvenate Modular Hip System Design Features . 8Table 2. Rejuvenate Modular Hip System Achieved Neck Length/Stem Compatibility. 23Table 3. Rejuvenate Modular Hip System Head/Neck Combinations . 23Table 4. Primary THA Expected Complication Rates . 45CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page viiList of FiguresFigure 1. Adverse Event Decision Tree .30CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page viiiList of AbbreviationsADEAdverse Device EffectAEAdverse EventAPAnteroposteriorBMIBody Mass IndexCRFCase Report FormCSAClinical Study AssociateCSMClinical Study ManagerDCFData Clarification FormDCRData Clarification RequestECEthics CommitteeGCPGood Clinical PracticeHHSHarris Hip ScoreHIPAAHealth Insurance Portability and Accountability ActICMJEInternational Committee of Medical Journal EditorsIRBInstitutional Review BoardLEASLower Extremity Activity ScaleNIDJDNon-Inflammatory Degenerative Joint DiseasePERProduct Experience ReportPIPrimary InvestigatorQOLQuality of LifeROMRange of MotionSAESerious Adverse EventSCStudy CoordinatorSF-12Short Form-12THRTotal Hip ReplacementUADEUnanticipated Adverse Device EffectUHMWPEUltra High Molecular Weight PolyethyleneCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page 1Study SynopsisA prospective, post-market, multi-center study of the outcomes of theTitleRejuvenate Modular Hip SystemShort TitleRejuvenate Modular Outcomes StudyProtocol Number68PhasePost-marketThis study will be a prospective, non-randomized evaluation of theRejuvenate Modular Hip System for primary total hip replacement(THR) with a cementless application in a consecutive series ofMethodologypatients who meet the eligibility criteria.Data from Secur-Fit HA monolithic femoral stem cases that areenrolled in the Trident X3 Polyethylene Insert Study will be used asa historical control group. Minimum functional evaluation, assessment of health relatedquality of life (QOL) and radiographic follow-up of eachprimary THR case to 5 yearsStudy Duration Enrollment period of 24 months Completion of pain, satisfaction and survivorship patientquestionnaire between 6 and 10 years Study Center(s)Approximate 12-year total duration10 – 12The success rate, defined as freedom from femoral stem/neckconstruct revision/removal for any reason, for hips implanted with theHypothesisRejuvenate Modular Hip System, is no worse than for hipsimplanted with the Secur-Fit HA monolithic femoral hip stem at 5years postoperative.CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page 2Primary: To evaluate the success rate of cementless primary THR withthe Rejuvenate Modular Hip System as compared to theSecur-Fit HA monolithic femoral hip stem, through absenceof revision at 5 years postoperative.Secondary: To obtain natural preoperative biomechanical measurementsand compare a preoperative plan to postoperativebiomechanical measurements, within the Rejuvenate Modular Hip System group. To compare pain, function and health related QOL betweenthe Rejuvenate Modular Hip System group and the Secur-ObjectivesFit HA monolithic femoral stem group. The followingoutcomes measures will be used for this comparison: oHarris Hip Score (HHS)oShort Form-12 (SF-12)oLower Extremity Activity Scale (LEAS)To review radiographic stability and complications betweenthose implanted with the Rejuvenate Modular Hip Systemand the Secur-Fit HA monolithic femoral stem group.Published complication rates with similar devices as well ascomplications related to modularity will be reviewed, asapplicable.Number of Subjects 240 casesCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page 3Inclusions:A. Patient has signed an IRB approved, study specific InformedPatient Consent Form.B. Patient is a male or non-pregnant female age 18 years orolder at time of study device implantation.C. Patient has primary diagnosis of Non-InflammatoryDegenerative Joint Disease (NIDJD).D. Patient is a candidate for a primary cementless total hipreplacement.E. Patient is willing and able to comply with postoperativescheduled clinical and radiographic evaluations andrehabilitation.F. Patient’s operative femur templates to Rejuvenate ModularStem size 7-12.Diagnosis and Main Exclusions:Inclusion/ExclusionG. Patient has a Body Mass Index (BMI) 40.CriteriaH. Patient has an active or suspected latent infection in or aboutthe affected hip joint at time of study device implantation.I.Patient has a neuromuscular or neurosensory deficiency,which limits the ability to evaluate the safety and efficacy ofthe device.J. Patient is diagnosed with a systemic disease (e.g. LupusErythematosus) or a metabolic disorder (e.g. Paget’sDisease) leading to progressive bone deterioration.K. Patient is immunologically suppressed or receiving steroids inexcess of normal physiological requirements(e.g. 30 days).L. Patient requires revision surgery of a previously implantedtotal hip replacement or hip fusion to the affected joint.M. Patient has a known sensitivity to device materials.N. Patient is a prisoner.CONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page 4Rejuvenate Modular Hip SystemRequired Components:Study Device Rejuvenate Modular Stem Rejuvenate Modular NeckThe femoral components must be used in a cementless application.Reference TherapySecur-Fit HA Monolithic Femoral Hip StemStryker acetabular components and femoral bearing heads must beused according to this study protocol. The following ancillary devicesare permissible: Stryker Trident or Tritanium Primary acetabular shells, with orwithout screw fixation, as well as Stryker Restoration ADMAncillary Devicesacetabular shells Stryker alumina ceramic inserts (for use with Trident acetabularshells) or X3 inserts Stryker LFIT CoCr heads; delta ceramic heads; or aluminaceramic heads with adaptor sleeveCONFIDENTIALThis material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker.
Rejuvenate Modular Outcomes StudyStryker Orthopaedics Clinical Study ProtocolVersion 1.0Page 5Primary:The 90% confidence interval around the difference in success rate(Rejuvenate Modular Hip System – Secur-Fit HA monolithicfemoral stem) will be computed at 5 years postoperative. For thenon-inferiority comparison, the lower bound of this 90% confidenceinterval will be compared with -5%.Secondary: A t-test or Wilcoxon test will be used to co
This material is the property of Stryker Orthopaedics. Do not disclose or use except as authorized in writing by Stryker. Objectives Primary: To evaluate the success rate of cementless primary THR with the Rejuvenate Modular Hip System as compared to the Secur-Fit