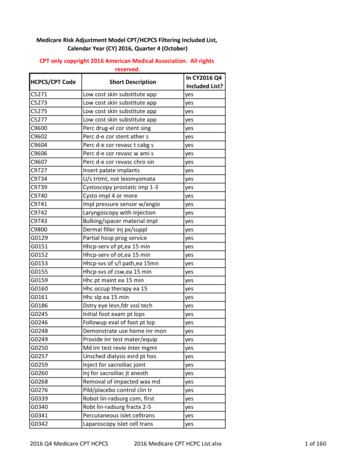
Transcription
CPT Editorial Summary of Panel ActionsFebruary 2016CPT Editorial Summary of Panel Action February, 2016Please be aware that this action is a reflection of the discussion at the most recent Panel meeting. Disclosure ofPanel action and deliberation is limited to the information contained in this Summary of Actions. Premature releaseof coding information other than that contained in this document is prohibited under the CPT ConfidentialityAgreement. Codes are not assigned, nor exact wording finalized, until just prior to publication. Release of morespecific CPT code set information is timed with the release of the entire set of coding changes in the CPTpublication.If an applicant or other interested party believes an action of the CPT Editorial Panel was in error, that individual orentity may request reconsideration of the Panel action. An “interested party” is an individual or entity that maypotentially be impacted by the Panel’s decision, regardless of whether they participated in the Panel’s originalconsideration of the matter.Submitting the Request: Requests for reconsideration must be received by AMA staff no later than midnight,Central, March 24, 2016 fourteen (14) business days after the published posting date (March 4, 2016) of theSummary Grid of Editorial Panel Actions on the CPT website www.ama-assn.org/go/cpt. The request should contain(1) the specific action requested for reconsideration; (2) the basis for the reconsideration request; and (3) allinformation relevant to the matter, including any literature (whether favorable or adverse) related to the requestor’sposition. Requests for reconsideration and relevant information must be in writing and submitted to:Marie MindemanDirector, CPT Coding, Editorial and Regulatory ServicesAmerican Medical AssociationAMA Plaza330 N. Wabash Ave., Suite 39300Chicago, IL 60611-5885Participation by Interested Parties: The receipt of a request for reconsideration, the identity of the party seekingsuch, and a brief summary of the basis for the reconsideration request will be noted in the summary grid of EditorialPanel actions for the agenda item. The applicant and interested parties are responsible for monitoring postings tothe CPT website with respect to requests for reconsideration. CPT staff will make reasonable efforts to identifypotentially interested parties and notify them of the receipt of the request for reconsideration and the opportunity tobe heard. An interested party seeking to comment on the request for reconsideration should submit its commentswithin fourteen (14) days of the posting of the notice (see deadline in Submitting the Request above) in thesummary grid of Editorial Panel actions that a request for reconsideration has been received. Comments shouldinclude (i) a statement of the nature of the commenter’s interest in the issue, (ii) the specific comment and reasonfor the comment, and (iii) all relevant material including any literature (whether favorable or adverse) related to thecommenter’s position. Comments should be submitted to the Director of CPT Editorial Research & Development atthe address shown above. The applicant(s) who submitted the original code change proposal is automaticallyconsidered an interested party and will receive notice of any request for reconsideration submitted by another party.Note: Codes that contain an 'X' (e.g., 1002X4, 234X2X, 0301XT) are placeholder codes that are intended, throughthe first three digits, to give readers an idea of the proposed placement in the code set of the potential codechanges. These codes are not used for claims reporting and will be removed and not retained when the final CPTDatafiles are distributed on August 31st of each year. To report the services for 'X' codes, please refer to the actualcodes as they appear in the CPT Datafiles publication distributed on or before August 31st of each year.Copyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016
Tab #NameCodesDescription ofEditorial Panel Action6Management of Single HighRisk Disease WITHDRAWN------ ------------ ------7Behavioral Health CareManagement Services994X1994X2994X3Accepted addition ofcodes 994X1, 994X2,994X3 for psychiatriccollaborative caremanagement.------ ------------ ------(Psychiatric Collaborativecare Management)8Medication TherapyManagement Services WITHDRAWN9Chronic Care ManagementServices10Unusual ComplexityEvaluation and ManagementServices11Acute Care ManagementNursing Facility WITHDRAWN12Non-Face-to-Face AcuteCare13Cognitive ImpairmentAssessment and Care PlanServices99XX3Accepted addition of99XX3 for reportingassessment and careplan services for patientswith cognitiveimpairment.14Aspiration of Breast FluidNipple - WITHDRAWN------ ------------ ------15Exclusionary Note Revisionsin Surgery RadiologyCategory ht 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016PostponedRejected------ ------------ ------RejectedAccepted additionrevision, and deletion ofexclusionaryparenthetical instructionto provide more accuratecoding instructions
Tab #NameCodesDescription ofEditorial Panel Action16Diagnostic Bone MarrowAspiration and Biopsy382X33822038221Accepted revision ofcodes 38220, 38221 forclarification to include theterm “diagnostic”;addition of code 382X3,and instructions to reportdiagnostic biopsy andaspiration17Ligation of Vas Deferens Delete 5545055450Accepted deletion ofcode 5545018PercutaneousNeurostimulator Placement19Incision-Implantation ofRespiratory Sensing Lead WITHDRAWN------ ------------ ------20Chest 710X1-710X4Accepted deletion of71010-71035; andaddition of codes710X1-710X421Abdominal X-ray74000740107402074022740X1740X2740X3Accepted deletion ofthree abdomen-specific Xray codes 74000, 74010,74020 and to replacethem with three newcodes 740X1, 740X2,740X3.22Drug epted addition ofcodes 803X1-803X3;revision of guidelines forreporting presumptivedrug testing; deletion ofcodes 80300-80304; anddeletion of Drug Classlistings and Examples23Definitive Drug ScreenMatrix Assisted LaserDesorption Ionization-Timeof Flight – WITHDRAWN------ ------------ ------Copyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016Postponed
Tab #NameCodesDescription ofEditorial Panel Action24Tier 1 for HaptoglobinGenotype Assay WITHDRAWN------ ------------ ------25Tier 1 SEPT9 MethylationAnalysis for ColorectalCancer813X781401Accepted addition ofMolecular Pathology Tier1 code 813X7; revisionof Tier 2 code 81401.26GSP-Fetal MicrodeletionAnalysis for DiGeorgeSyndrome814X3Accepted addition ofcode 814X3 to reportgenomic sequenceanalysis of fetalchromosomalmicrodeletions incirculating cell-free fetalDNA in maternal blood27Tier 2 RNA Expression forMelanoma - WITHDRAWN------ ------------ ------28MAAA-Ovarian OncologyExclusionary Note Revision81503Accepted revision ofexclusionaryparenthetical notefollowing code 81503.29MAAA-Uveal MelanomaMetastatic Risk WITHDRAWN------ ------------ ------30MAAA-Follicle StimulatingHormone and HumanEpididymis 4 - WITHDRAWN------ ------------ ------31MAAA-ComprehensivePharmacogenetic Analysis WITHDRAWN------ ------------ ------32MAAA-Cutaneous MelanomaMetastisis Risk WITHDRAWN------ ------------ ------33Targeted GenomicSequence Analysis-8144581450 8145534Microdissection-Delete88380 88381 WITHDRAWNCopyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016Accepted revision ofthe Molecular Pathologyguidelines; addition ofthe definition of copynumber variants------ ------------ ------
Tab #NameCodesDescription ofEditorial Panel Action35TumorImmunohistochemistry88360 - WITHDRAWN------ ------------ ------36Infectious Agent Detection878XXAccepted addition ofcode 878XX to reportcentral nervous system37Quadrivalent InfluenzaVaccine Age 6 Months orOlder38Quadrivalent InfluenzaVaccine Cell CulturedDerived90661906X2Accepted addition ofcode 906X2 forquadrivalent derivedinfluenza virus vaccinefrom cell cultures; andrevision of code 90661to clarify that itrepresents a trivalentvaccine.39Pulmonary Diagnostic Tests9462094621946X2946X3Accepted deletion ofcode 94620; revision of94621; and addition ofcodes 946X2, 942X3 forexercise testing forbronchospasm40Medication TherapyPrescriber CollaborationService - WITHDRAWN------ ------------ ------41Auditory Function Evaluation92620 92621 ExclusionaryNote Revision WITHDRAWN------ ------------ ------42Cat III EndoscopicPlacement of Drug ElutingImplant - WITHDRAWN------ ------------ ------43Category III 8T0299T0300T0301T0302TCopyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2T0313T0314T0315T0316T0317TAccepted retention ofCategory III codes,0295T, 0296T.0297T0298T, 0312T, 0313T,0314T, 0315T, 0316T,0317TAccepted archiving ofCategory III code 0178T,0179T, 0180T, 0293T,0294T, 0299T, 0300T,0301T, 0302T, 0303T,0304T, 0305T, 0306T,0307T, 0309T, 0310T
Tab #NameCodesDescription ofEditorial Panel Action44Cat III SubcutaneousGlucose Sensor04X1T04X2T04X3TAccepted addition ofCategory III codes04X1T, 04X2T, and04X3T for subcutaneouscontinuous infusion45Category III Visual AxisAlignment for Centration WITHDRAWN------ ------------ ------46Cat III Insertion of AqueousDrainage Device0X46T0X47TAccepted addition ofCategory III codes0X46T, 0X47T for anaqueous drainage devicefor the subconjunctivalspace to lowerintraocular pressure.47Cat III 6T0X17T0X18T0X20T0X21T0X22T0X23T48MAAA Admin-ProstateCancer Prognosis WITHDRAWN------ ------49Telehealth Services ModifierAccepted addition of amodifier to facilitatereporting synchronousTelehealth services.50Code Set MaintenancePostponed51Telehealth ServicesAppendix ZZAccepted addition ofAppendix ZZ as a listingof CPT codes that maybe used for reportingsynchronous TelehealthServicesCopyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 20160X24TAccepted addition ofCategory III codes 0X11T– 0X24T for servicesrelated to insertion andmaintenance ofimplantable aorticcounterpulsationventricular assistsystems (iVAS).------ ------
Tab #NameCodesDescription ofEditorial Panel Action52Telehealth Services-RemoteEvaluation and ManagementServices - WITHDRAWN------ ------------ ------53Proprietary Lab Analyses54Definitive Testing of 4 orMore Drugs-Drug ClassesEC-BInpatient Neonatal andPediatric Critical CareGuideline Clarification992919929299468-99476Not accepted, No ActionTakenEC-COPKO 4K Score TestDescriptor0010MAccepted revision of thecode descriptor foradministrative code0010MEC-DPhase Out Process toRevise Category II CodesEC-ERequest forReconsiderationMyomectomy CodeExclusions for New Code585X1‒ LaparoscopicRadiofrequency Ablation ofUterine Fibroids585X15854558546Not Accepted forReconsideration, NoAction TakenEC-FRequest forReconsiderationTranscutaneous DopplerUltrasound Cardiac OutputMonitoring (TDM)9337X6Not Accepted forReconsideration, NoAction TakenEC-GVaccine Coding CaucusRequests ‒ InfluenzaVaccines and CCP Edits –Issue #1Copyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016Accepted the creation ofa Proprietary LaboratoryAnalyses (PLA) codechange application.PostponedNo Action TakenAcceptedrecommendation topublish a websiteannouncement regardingredefinition of theinfluenza vaccine codes.
Tab #NameCodesDescription of EditorialPanel ActionEC-GVaccine Coding CaucusRequests ‒ InfluenzaVaccines and CCP Edits –Issues #2-4Acceptedrecommendation torevise to the CPT CodeChange ApplicationEC-HSubmitted Literature Markedas ConfidentialAcceptedrecommendation torevise to the CPT CodeChange ApplicationEC-ILiterature Requirements ‒Editorial RevisionsIssues #1-4EC-ILiterature Requirements ‒Editorial RevisionsIssue #5EC-JRUC Report- Issue #1Electronic Analysis ofImplanted NeurostimulatorPulse Generator (9597095982)InformationalEC-JRUC Report- Issue #2Biopsy of Skin Lesion(11100 &11101)InformationalEC-JRUC Report- Issue #3InformationalAcceptedrecommendation to clarifythe Literaturerequirements for the CPTCode Change ApplicationNo Action TakenPulmonary Stress Test(94620)EC-JRUC Report- Issue #4Esophagectomy (432X5432X7)No Action TakenEC-JRUC Report- Issue #5Electroretinography (92275)InformationalEC-JRUC Report- Issue #6Non-invasive PhysiologicStudies of Extremity Veins(93965)InformationalCopyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016
Tab #NameEC-JRUC Report- Issue #7Central Nervous SystemAssessments/Tests Codes(96101, 96116, 96118)InformationalEC-JRUC Report- Issue #8Bone Marrow Aspiration andBiopsy (38220, 38221, &G0364)InformationalEC-KCPT Assistant EditorialBoard ReportIssues #1-2InformationalEC-LApplication FAQAcceptedrecommendation todevelop a new frequentlyasked question regardingthe CPT processEC-MNon-Staff RepresentationGuidelinesAcceptedrecommendation that acode change applicationbe generated for nonstaff representationguidelines for May 2016EC-NEsophageal MotilitySundownNot Accepted forReconsideration, NoAction TakenCopyright 2016, American Medical Association, All rights ReservedCPT Is a registered trademark of the American Medical AssociationUpdated March 4, 2016CodesDescription of EditorialPanel Action
Updated March 4, 2016 CPT Editorial Summary of Panel Actions . specific CPT code set information is timed with the release of the entire set of coding changes in the CPT publication. If an applicant or other interested party believes an action of the CPT Editorial Panel was in error, that individual or .