Transcription
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022In this documentDrugs that require prior authorization by AIM . 2Drugs that no longer require prior authorization by AIM . 6Blue Cross and BCN commercial preferred oncology drugs . 6Prior authorization for medical oncology and supportive care drugs is required through AIM Specialty Health for: Blue Cross commercial All fully insured members. This includes MESSA members, effective Jan. 1, 2022. Select self-funded groups, including UAW Retiree Medical Benefits Trust. To determine which groups have opted in and the date onwhich they opted in, see the document titled AIM medical oncology prior authorization program opt-in list for Blue Cross commercialself-funded groups.Note: For Blue Cross commercial members who have coverage through the UAW Retiree Medical Benefits Trust, see the Medicaloncology prior authorization list for UAW Retiree Medical Benefits Trust PPO non-Medicare members. All BCN commercial membersFor BCN commercial members, some drugs also have site-of-care requirements.You must submit authorization requests to AIM prior to administering any of the drugs on this list for those drugs to be eligible for payment.The medical oncology drug management program applies only to drugs prescribed for oncology diagnoses.Note: When prescribing these drugs for non-oncology diagnoses, don’t submit prior authorization requests to AIM. Instead: For Blue Cross commercial members: Fax all clinical documentation to the Pharmacy Clinical Help Desk at 1-866-915-9187. For BCN commercial members: Fax all clinical documentation to the Pharmacy Clinical Help Desk at 1-877-402-7695.1
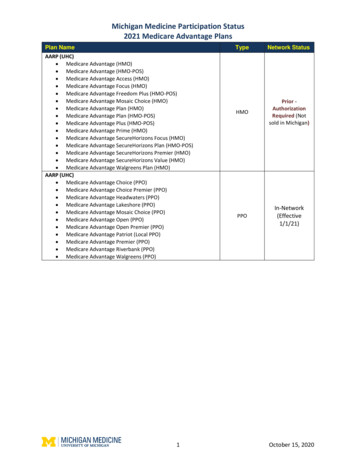
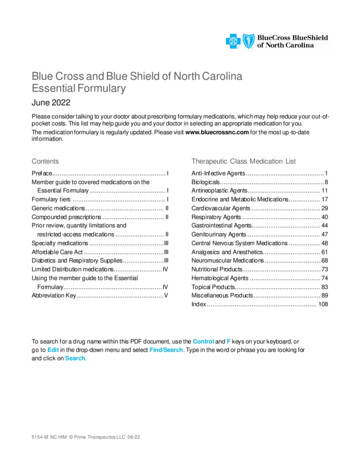
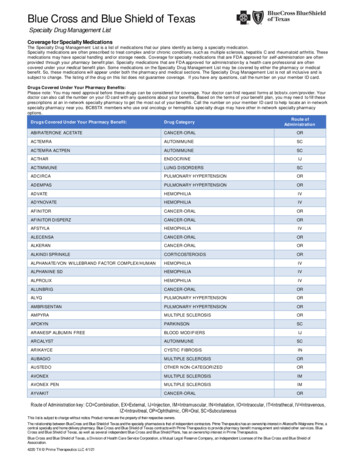
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022Drugs that require prior authorization by AIMBCN commercialeffective datesHCPCS codeBrand nameGeneric namePriorauthorizationSite of careBlue Cross commercialprior authorizationeffective date(1)J9264Abraxanepaclitaxel protein-bound particles8/1/201912/1/2020J9042Adcetris brentuximab vedotin8/1/201912/1/2020J9305Alimtapemetrexed disodium8/1/201912/1/2020J9057Aliqopa copanlisib 12/1/2020J9118Asparlas calaspargase /1/2019J9036Belrapzo bendamustine hcl11/1/201912/1/2020J9034Bendekabendamustine hcl8/1/201912/1/2020J9229Besponsa inotuzumab ozogamicin8/1/201912/1/2020J9037Blenrep belantamab mafodotin-blmf11/20/20201/18/2021J9039Blincyto blinatumomab8/1/201912/1/2020J1448Cosela trilaciclib5/24/20215/24/2021J9308Cyramza ramucirumab8/1/201912/1/2020J9348Danyelza naxitamab-gqgk4/22/20214/22/2021J9145Darzalex daratumumab8/1/201912/1/2020J9144Darzalex Faspro daratumumab and hyaluronidase-fihj7/24/202012/1/2020Q2050Doxil doxorubicin liposomal8/1/201912/1/2020J9269Elzonris tagraxofusp-erzs11/1/201912/1/2020J9176Empliciti elotuzumab8/1/201912/1/2020J9358Enhertu fam-trastuzumab deruxtecan-nxki3/2/202012/1/2020 9/1/202212/1/20202
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022BCN commercialeffective datesHCPCS codeBrand nameJ9055Erbitux J9246Generic namePriorauthorizationSite of careBlue Cross commercialprior authorizationeffective /1/201912/1/2020Q5108Fulphila pegflgrastim-jmdb4/1/20224/1/2022J9331Fyarro sirolimus protein-bound particles8/16/20228/16/2022J9301Gazyva /1/201912/1/2020J9356Herceptin Hylecta trastuzumab and rvalumab8/1/2019J9325Imlygic talimogene laherparepvec8/24/202012/1/2020J9319, a ixabepilone8/1/201912/1/2020J9281Jelmyto mitomycin7/24/202012/1/2020J9272Jemperli zitaxel8/1/201912/1/2020J9354Kadcyla ado-trastuzumab8/1/201912/1/2020Q5117Kanjinti trastuzumab-anns11/1/201912/1/2020J9271Keytruda pembrolizumab8/1/2019J0642Khapzory levoleucovorin8/1/201912/1/2020J9274Kimmtrak filzomib8/1/201912/1/2020J2820Leukine b-rwic10/1/2019 03
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022BCN commercialeffective datesHCPCS codeBrand nameGeneric namePriorauthorizationSite of careBlue Cross commercialprior authorizationeffective date(1)Q2049Lipodox doxorubicin liposomal8/1/201912/1/2020J9313Lumoxitimoxetumomab pasudotox-tdfk10/1/201912/1/2020J9353Margenza asitamab-cxix11/20/20201/18/2021J2562Mozobil plerixafor8/1/201912/1/2020Q5107Mvasi bevacizumab-awwb8/1/201912/1/2020J9203Mylotarg gemtuzumab ozogamicin8/1/201912/1/2020J2506Neulasta ;Neulasta OnPro pegfilgrastim8/1/201912/1/2020Q5110Nivestym filgrastim-aafi8/1/20194/1/2021J9205Onivyde irinotecan liposome8/1/201912/1/2020J9299Opdivo nivolumab8/1/2019J9298Opdualag nivolumab and relatlimab-rmbw12/1/202212/1/2022J9177Padcev enfortumab vedotin-ejfv3/2/202012/1/2020J9306Perjeta pertuzumab8/1/201912/1/2020J9316Phesgo pertuzumab, trastuzumab vy polatuzumab mumab8/1/201912/1/2020J9204Poteligeo puleucel-t8/1/201912/1/2020J9311Rituxan Hycela rituximab-hyaluronidase human8/1/201912/1/2020J9061Rybrevant amivantamab-vmjw9/27/20219/27/2021 9/1/202212/1/20204
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022BCN commercialeffective datesHCPCS codeBrand nameGeneric namePriorauthorizationSite of careBlue Cross commercialprior authorizationeffective date(1)J9227Sarclisa ximab8/1/201912/1/2020J9022Tecentriq atezolizumab8/1/2019J9273Tivdaktisotumab vedotin-tftv5/23/20225/23/2022Q5116Trazimera amustine hcl8/1/201912/1/2020J9317Trodelvy sacituzumab govitecan-hziy7/24/202012/1/2020J3490, J3590, 03Vectibix panitumumab8/1/201912/1/2020J9228Yervoy ipilimumab8/1/2019J9352Yondelis trabectedin8/1/201912/1/2020J9400Zaltrap ziv-aflibercept8/1/201912/1/2020Q5101Zarxio filgrastim-sndz8/1/20194/1/2021J9223Zepzelca lurbinectedin9/25/202012/1/2020Q5120Ziextenzo pegfilgrastim-bmez4/1/20224/1/2022Q5118Zirabev bevacizumab-bvzr11/1/201912/1/2020J9359Zynlonta loncastuximab tesirine-lpyl7/26/20217/26/2021 9/1/20229/1/202212/1/202012/1/2020For drugs with effective dates prior to Jan. 1, 2022, the prior authorization requirement is effective for MESSA members for dates of service on or afterJan. 1, 2022. To view a list of self-funded groups that have opted in to this program and the prior authorization effective dates for those groups, see thedocument titled AIM medical oncology prior authorization program opt-in list for Blue Cross commercial self-funded groups.(1)5
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, 2022Drugs that no longer require prior authorization by AIMHCPCScodeCommercialplanDrugPrior authorization requirementStart dateEnd dateReasonJ9245melphalan (Evomela )BCN8/1/20196/30/2020HCPCS code changed to J9246 on 7/1/2020J9247melphalan flufenamide(Pepaxto )BCN/BlueCross5/24/202111/15/2021Market withdrawalJ2505pegfilgrastim (Neulasta )BCN8/1/201912/31/2021HCPCS code changed to J2506 on 1/1/2022J2505pegfilgrastim (Neulasta )Blue Cross12/1/202012/31/2021HCPCS code changed to J2506 on 1/1/2022J0641levoleucovorin (Fusilev )BCN/BlueCross8/1/20195/31/2022Market withdrawalBlue Cross and BCN commercial preferred oncology uctStart dateAuthorizationrequiredSubmit requestthroughQ5107bevacizumab-awwb (Mvasi )X4/1/2021XAIMQ5118bevacizumab-bvzr (Zirabev )X4/1/2021XAIMJ9035bevacizumab (Avastin )X4/1/2021XNovoLogixC9142bevacizumab-maly (Alymsys )X8/25/2022XNovoLogixQ5117trastuzumab-anns (Kanjinti )X4/1/2021XAIMQ5116trastuzumab-gyyp (Trazimera )X4/1/2021XAIMJ9355trastuzumab (Herceptin )X4/1/2021XNovoLogixQ5113trastuzumab-pkrb (Herzuma )X4/1/2021XNovoLogixQ5114trastuzumab-dkst (Ogivri )X4/1/2021XNovoLogixQ5112trastuzumab-dttb (Ontruzant )X4/1/2021XNovoLogix 6
Medical oncology prior authorization list forBlue Cross and BCN commercial membersMedications that require authorization by AIM Specialty Health Revised Sept. 1, ctStart dateAuthorizationrequiredSubmit requestthroughJ2506pegfilgrastim (Neulasta /Neulasta Onpro )X4/1/2021XAIMQ5120pegfilgrastim-bmez (Ziextenzo )X4/1/2022XAIMQ5108pegfilgrastim-jmdb (Fulphila )X4/1/2022XAIMQ5122pegfilgrastim-apgf (Nyvepria )X4/1/2022XNovologixQ5111pegfilgrastim-cbqv (Udenyca )X4/1/2021XNovoLogixQ5119rituximab-pvvr (Ruxience )X4/1/2021Q5123rituximab-arrx (Riabni )X4/1/2021J9312rituximab (Rituxan )Q5115rituximab-abbs (Truxima )Q5101filgrastim-sndz (Zarxio )Q5110filgrastim-aafi (Nivestym )J1447tbo-filgrastim (Granix )J1442filgrastim (Neupogen )Q5125filgrastim-ayow (Releuko ) oLogixX11/1/2022XNovoLogix AIM Specialty Health is an independent company that manages authorizations of select services for Blue Cross Blue Shield of Michigan and Blue CareNetwork.7
Medical oncology prior authorization list for Blue Cross and BCN commercial members . Medications that require authorization by AIM Specialty Health Revised Aug. 1, 2022 . 2 . Drugs that require prior authorization by AIM . HCPCS code Brand name Generic name BCN commercial effective dates Blue Cross commercial prior authorization effective .