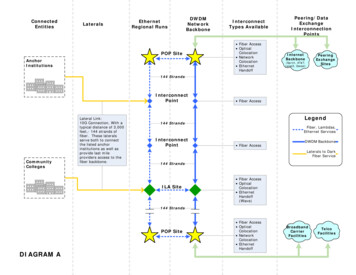
Transcription
Life Sciences & HealthcareReviewing Hot Regulatory Challenges,New EU GDP “Good Distribution Practices”Mike MeakinVice President – Global Quality, Regulatory & ComplianceLife Sciences & HealthcareLongboat Key - 11th October 2012
IntroductionLife Sciences & HealthcareLogistics Changes – global enlargement, manufacturing, patents, volumes, litigationRegulatory Trends GDPs evolving into GMPs – Harmonisation ICH & PIC/s GCCMP - TTSPP Time Temperature-Sensitive Pharmaceutical Product (WHO TRS 961) QRM (ICH Q9 & ISO 14971) – Risk based Approach Supply Chain Integrity – SSFFC – Counterfeits, Theft, Diversion, Adulteration, Expiry Licensing, Contractors, Bona FidesChallenges: Regulation – greater and greater regulation – increase costs Infrastructure – Pharma grade – Plants & Facilities HVAC Capacity – SISPQ cross contamination ValidationGrowing Markets – BRIC-TM Importing countries Exporting countriesCompliance & Patient Safety First!Longboat Key 11th October 2012Page
DHL Life Sciences GxP FootprintLife Sciences & HealthcareDHL has over150 Life Sciences &Healthcare facilities GloballyOver 100 warehouses operating to GxP requirementsGMP Facilities Pharma & ISO 13485Compliance & Patient Safety First!Longboat Key 11th October 2012Page
Life Science & Healthcare Quality Systems - Patient Safety!Life Sciences & HealthcareQuality objectives (DRA, GAMP, GDP)Patient safety Protecting the patient – mistakes can harm even kill!Product quality GMP compliant must have SISPQ characteristicsCompliance Customer quality assurance agreementsHistory – result more legislation1898 – Heroin marketed for coughs! - Harrisons Tax Act 19141902 – Biologics Control Act – 22 children from contaminated vaccines1906 – FDA formed1937 – Elixir Sulfanilamide tragedy1939 – Faddis MF. Eliminating errors in medications.1940 – Winthrop Sulfathiazole – Phenobarbital contamination1961 – Thalidomide withdrawn – Drug Amendment Act 19621972 – Fatalities Devonport Hospital Lot D1192/C Evans Medical1982 – Tylenol laced with Cyanide in Chicago (tamper resistant)2000 – To Err is Human – Bill Clinton2008 – Melamine adulteration 6 babies die 860 hospitalized – 2 executions!2009 – Heparin recall – 1800 adverse events 246 deaths - 86 fit profile** 12 Chinese companies supplied 11 countriesCompliance & Patient Safety First!2011 – Isotab (isosorbide mononitrate) c150 deaths 450 serious ARsLongboat Key 11th October 2012Page4
GDP - Robust recall process – understanding PDA TR 55Life Sciences & HealthcareHeat Treated Mark Epal PalletNON HT Epal* PalletHeat Treated Mark CHEP** Pallet*Epal – European Pallet Association**CHEP - Commonwealth Handling Equipment PoolCompliance & Patient Safety First!Longboat Key 11th October 2012Page
Understanding the need for GDP?Compliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage6
LSPs also have commercial and legal challengesCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage
Contamination Issues What can Go WrongCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage8
Can we trust suppliers - Counterfeiters Paradise?Compliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage9
Regulatory Changes in many Countries including BRIC-TMWith over 30 different GDP Worldwide and several recent updates,the regulatory world is also changingLife Sciences & HealthcareAS OF SEPT, 2012BRIC-TMl ChangesSupply Chain Integrity/GCCMPNew EU GDPFocus on EU GDPDHL GxP PolicyChina, India, Turkey,South KoreaArgentina, Australia,Canada, Czech, Egypt,Ireland, Italy, Romania,Malaysia, Saudi Arabia,UAE, Singapore, SouthAfrica, VenezuelaBrazil, MexicoPROCESS – PDA Technical SupportSTANDARDS No 39 Revised 2007 T-C Medicinal PrdouctsNo 46 Issued 2009 – GDP last mileNo 52 Issued 2011 – GDP Pharma Supply ChainNo 53 Issued 2011 – Stability TestingNo 55 Issued 2012 – Detect/Mitigate TBA/TCANo 58 Issued 2012 – Risk Management TCDCompliance & Patient Safety First!IATA Labeling StandardNew PDA Guidance coming outLongboat Key 11th October 2012USP 1079 Good Storage & Shipping PracticesUSP 1083 draft – GDP Supply Chain IntegrityIATA PCR Ch 17 – T&TS Label 1 July 2012AFNOR NF S99-700, Oct 2009ASTM D3103 – Thermal Insulated PerformanceISTA 7D & 7E – Thermal Transport PackagingPage 10
New EU GDP Guidelines: 30 Worldwide GDPs –PIC/s looking at new EU GDP as a Global standardCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage 11
Updated and Newly Issued Regulations and GuidelinesCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage 12
DHL Yellow Book – Annex K results on GDP issuesLife Sciences & HealthcareAnnex K: DHL Life Sciences & Healthcare GxP Audit ToolCompliance & Patient Safety First!Longboat Key 11th October 2012Page
Challenges meeting GSP & GDPCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage
New EU GDP Guidelines – GMP GDPGMPs Continue to Evolve into GDP & GWPLife Sciences & HealthcareGDP QMS SCICompliance & Patient Safety First!Longboat Key 11th October 2012Page 15
Other Implications Directive 2011/62 amending 2001/83Life Sciences & Healthcare Falsified Medicines Directive andunique identifiers having a impacton Good Traceability Practice USP 1083 – GDP – Supply ChainIntegrity is doing something similarin the USA linking USP 1079 PDA TR 52 – is showing how toassess logistics providers PIC/s – have GDP on their 2015Road map may also adopt EU GDP Impact pedigree and serialisationfinish with a few pictures and ashort videoCompliance & Patient Safety First!Longboat Key 11th October 2012Page 16
DHL responded to the EU GDP in Dec 11- Over 20 specific comments in 25 pagesCompliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage 17
Life Sciences & HealthcareProposed new EU GDP Chapters 1-3Chapter 1:Quality Management: A QMS has to be in place Deviation Reporting, CAPA, Change Control QRM intorduced based on ICH Q9Chapter 2 Personnel: Responsible Person in each distribution point (permanence) Delegation possible ? Documented deputyships for other personnel Hygiene & TrainingChapter 3: Premises and Equipment: Temperature & environment control – Temp Mapping Qualification & validation CSV – Computer Systems Validation18Compliance & Patient Safety First!Longboat Key 11th October 2012Page
LSPs - Qualified People - Chapter 2 GDP & GMPMike MeakinDHL employs over 60 dedicated fulltime Pharmacists globallyWorkforce 5% dedicated QAVP - Global QualityRegulatory & ComplianceRatios 1:50 GDP 1:30 GMPLife Sciences & HealthcareKylie CoulsonBronagh McVeigh 9 14AUSTRALIATom Young –Farakh Rashid –Ashraf LucaSarah MansourMarie Naim MorcosEGYPTLife Sciences & HealthcareValidation ManagerQA/RA ManagerGlobal HealthcareHealthcare EMEAEstelle HarrisReshma ChunderYolanda van VuurenMalani MoodleyVerna Crouse (Cons)Binu PanickerIndrajit SarkarUAESOUTH AFRICASally VerdurmenDirk LedegenFrancoise RogisterGaellle BlondeauAlain WarnierMartina ImmelKristin VandenbusscheBELGIUMFernanda TelesRodrigo Bianconi RAFernanda Lago QALucimeire Sola CSVJuliana S. Filizzola 15 PharmacistsBRAZILChloe ShihAileen ZhuCHINAKatja NolviFinlandSuyog KudtarkarINDIAElodie HantzVincent BoudySylvain Moraud (V)FRANCEHikaru ShibaMizoumi OnukiNaoshi KonogayaTatsuhito HishidaSayaka MochizukiJAPANMiguel SanshezLuis Nunez - CainCoPANAMAKatarzyna GapskaArtur SpicakValerie ByrneIRELANDPOLANDAlfonso RoaLeticia Pena4 Responsible PersonsMEXICOWilfried HöraTorsten BaeumerGERMANYAlexandra KaracsMaria Assunta SgroErika GarganeseMargherita BubbicoRoberto BigagliAlberto Ugazio 3ITALYHUNGARYCompliance & Patient Safety First!Mark BenzahiaSalem Al SuhaimyNasser Al ShammryMohammed Al GhamdiSAUDI ARABIANETHERLANDSRUSSIALongboat Key 11th October 2012Juan CejudoSPAINKati LottonenChoi Wei ChaiYun NgFermin Joel BagadiongSINGAPOREJoana RibeiroPORTUGALAnja Verstegen 7Peter Klaessens WSCJay Rijnen QP ManufacturingCees Schaap QP- Distribution Evgenia EgorovaAmelia QuijanoRoberto Azcuaga 15Carol Candlish QPDavid Burnett QPDavid Stevens QPPaul SellwoodSWEDENAndrew WatkinsPete RhodesNiall SparePatrick TrainClaire MacintoshUNITED KINGDOMBernd ThomasSWITZERLANDJT KimYJ HurEphrem SongSOUTH KoreaBerna TuncelTURKEYMelissa MalesicKonni SynderAmy NicholsMonica Hinderer LicensesUSAPage 19
Life Sciences & HealthcareProposed new EU GDP Chapters 4-7Chapter 4: Documentation: Focus on record keeping and traceability of all transactions “batch numbers - safety features ?” Take the advantage of the electronic meansChapter 5: Operations: Qualification of Suppliers Qualification of Customers – Bona fides – deliver to licensed premises Good Storage Practice (reception, delivery, packing, destruction, export etc )Chapter 6: Complaints, Returns, Recalls, suspected Falsified Medicinal Products Complaints, Returns & RecallsChapter 7: Contract operations: QTAs Quality Technical Agreements between Sub contractors & Suppliers20Compliance & Patient Safety First!Longboat Key 11th October 2012Page
Life Sciences & HealthcareProposed new EU GDP Chapters 8-10Chapter 8: Self-inspections: Independence – auditing Subcontractors in accordnace with GDPChapter 9: Transportation: (most contentious being modified?) Tranportation (24 hours) Containers packaging and labelling Special condition – controoled substances and radio active medicines Temperature Control during transportChapter 10: Specific provisions for Brokers: Regulating the middlemen “They gain, they have to share the pain!” Brokers need authorisation – Quality Systems etc too21Compliance & Patient Safety First!Longboat Key 11th October 2012Page
Validated temperature-control systems –Refrigerated vehicles OQ & PQ undertaken in Climate Chambers?Compliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage
New EU GDP Guidelines:DHL Yellow Book – 4 AreasActioned in Version 6.19.19 Validated temperature-control systems (e.g.thermal packaging, temperature-controlledcontainers, and refrigerated vehicles) should beused to ensure correct transport conditions aremaintained between the distributor and customer.Customers should be provided with a temperaturedata to demonstrate that products remained withinthe required temperature storage conditions duringtransit, if requested.9.1 The required storage conditionsfor medicinal products should bemaintained during transportationwithin the defined limits asdescribed on the packaginginformationCompliance & Patient Safety First!Life Sciences & HealthcareLongboat Key 11th October 2012Page 23
Regulations, Guidelines & Customer Standards DevelopInto a LSP PQS Guidelines Includes cGMPLife Sciences & HealthcareCustomer requirements QAACompliance & Patient Safety First!Longboat Key 11th October 2012Page 24
It is going to cost more?Compliance & Patient Safety First!Longboat Key 11th October 2012Life Sciences & HealthcarePage 25
Compromised Integrity – Supply Chain ThreatsLife Sciences & HealthcareThe Falsified Medicines Directive has further requirementsThe US FDA refers to 5 areas of threat to SCI: Counterfeit drugs Cargo Theft/Stolen drugs Diverted drugs Internationally adulterated drugs (EMA) Expired drugsAll the above feed into the need for Pedigree or Serialisation andare linked with Supply Chain Integrity and new standards e.g. USP1083Compliance & Patient Safety First!Longboat Key 11th October 2012Page
Labeling - Challenging Regulatory Environment TraceabilityLife Sciences & HealthcareSwedish Trial 2009Key:Track & Trace Law in PlaceTrack & Trace Law discussedMachine-readable law in placeSerialisation law in placeSerialisation law discussedCompliance & Patient Safety First!Longboat Key 11th October 2012Page 27
Life Sciences & HealthcareCompliance & Patient Safety First!Longboat Key 11th October 2012Page 28
Life Sciences & HealthcareThank You - Contact DetailsMike MeakinVice PresidentGlobal Quality & Regulatory ComplianceDHL Supply ChainPhoneMobile 44 (0) 1327 308 400 44 (0) 7774 299 402mike.meakin@dhl.comCompliance & Patient Safety First!Newnham DriveHeartlands Business ParkDaventryNN11 8YGUnited KingdomLongboat Key 11th October 2012Page 29
ISTA 7D & 7E - Thermal Transport Packaging No 39 Revised 2007 T-C Medicinal Prdoucts No 46 Issued 2009 - GDP last mile No 52 Issued 2011 - GDP Pharma Supply Chain No 53 Issued 2011 - Stability Testing No 55 Issued 2012 - Detect/Mitigate TBA/TCA No 58 Issued 2012 - Risk Management TCD New PDA Guidance .