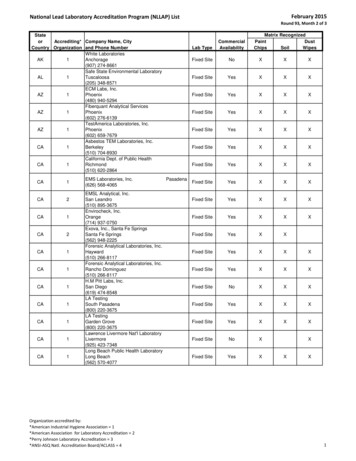
Transcription
Experience LaboratoryAccreditation ThatEvolves and ElevatesQuality to a DifferentStandardDr. Jeremy Hart, MD, MBA, FCAPPatricia Nortmann, MBA, MT (ASCP)Wednesday, April 13, 202212:00–1:00 PM CST College of American Pathologists.
Webinar HousekeepingToday’s webinaris beingrecorded College of American Pathologists.A link will beprovided so youcan revisit thecontent andshare it withcolleaguesWe invite you tointeract with thespeakers bysubmittingquestions in theAsk aQuestion boxTell us how wedid in the surveyimmediatelyfollowing thewebinar13 April 20222
Today’s PresentersExperience Laboratory Accreditation That Evolves andElevates Quality to a Different StandardDr. Jeremy Hart, MD, MBA, FCAPMedical Director of Clinical LaboratoriesSt. Elizabeth Healthcare College of American Pathologists.Patricia Nortmann, MBA, MT (ASCP)AVP Laboratory ServicesSt. Elizabeth Healthcare13 April 20223
St. Elizabeth HealthcareMISSIONAs a Catholic healthcare ministry, we provide comprehensive andcompassionate care that improves the health of the people we serve.VISIONSt. Elizabeth will lead the communities we serve to become thehealthiest in America. College of American Pathologists.13 April 20224
About St. Elizabeth HealthcareSix facilities throughoutNorthern Kentucky &Southeastern IndianaCovingtonDearbornEdgewoodFlorence165 St. ElizabethPhysicians specialty andprimary care officeslocated throughoutKentuckyOhioIndianaFt. ThomasGrant College of American Pathologists.13 April 20225
Laboratories at St. Elizabeth Healthcare 10 CLIA certified laboratories “System” CAP accreditation Joint venture “core” laboratory 70 million annual operatingbudget 400 FTEs and 10 pathologists 6.6 million annual billable tests 19 outpatient draw sites College of American Pathologists.13 April 20226
Operating a High-Quality Laboratory Serviceat St. Elizabeth HealthcareToday’s Laboratory NeedsCAP LaboratoryAccreditation ProgramMaintain continuous qualityimprovements to strengthen qualitymanagementCustomized discipline-specificchecklists combined with peerinspectionsFocus on competency, learning,and developmentAccess to complimentary learningopportunities, tools, and resourcesAdapt to an evolving environmentwhile upholding safety for patientsand staffAnnual checklist updatesincorporate input from 33 scientificresource committees College of American Pathologists.13 April 20227
The College of American PathologistsThe Preferred Partner for Laboratory Excellence College of American Pathologists. College of American Pathologists.13 April 20228
Focused on Collaborationfrom Our Founding Largest member association ofboard-certified pathologistso 18,000 global members Exclusive laboratory focus Trusted partner of CMS, FDA, CDC,ASCO, NMDP, UNOS, and many others College of American Pathologists.13 April 20229
Leading in Laboratory QualitySolutions Accrediting nearly 8,000 laboratoriesworldwide with 500 abroad in 50 countries Providing 23,000 laboratories worldwidewith proficiency testing Offering cancer protocols for completeand accurate diagnostic reports College of American Pathologists.13 April 202210
CAP Leadership in the Pandemic Pathologists and laboratorianso Delivered in-depth guidance and tools on implementing SARS-CoV-2 testingin a rapidly evolving environment Laboratoryo Launched SARS-CoV-2 proficiency testing for viral and serology testso Successfully petitioned CMS to delay inspectionso Provided safety precautions for anatomic pathology procedures andautopsies plus recommendations for protecting the blood supply via ourresource committees Communityo Gave input to the AMA for development of new CPT codes and advocated forincreased reimbursement College of American Pathologists.13 April 202211
Accreditation That Goes Beyond ComplianceCOMPLYwith CLIA, FDA, andOSHA standardsSTAY CURRENTwith advances in laboratorymedicine, technology, regulations College of American Pathologists.EXCEED 40% of our standards exceedminimum requirementsconsequently bolstering patientcare and safetyPURSUElaboratory excellence withindustry best practices13 April 202212
Accreditation to Elevate Quality and Mitigate RiskCAP Laboratory Accreditation ProgramDiscipline-specific ChecklistsCollaborative Peer InspectionsEducational Resources College of American Pathologists.13 April 202213
A Blueprint for Running a High-QualityLaboratory Simplify compliance Provide best-in-class standards Guided by expertise from all laboratory participants Incorporate best practices Written in straightforward languageCAP AccreditationChecklists Updated annually Suitable for all staff College of American Pathologists.13 April 202214
Discipline-Specific ChecklistsFor guidance across the laboratory and at the bench levelDirector AssessmentLaboratory GeneralAll CommonHematology & CoagulationChemistry & ToxicologyUrinalysisMicrobiologyAnatomic PathologyCytopathologyCytogeneticsForensic Drug TestingClinical Biochemical GeneticsPoint of CareBiorepositoryFlow CytometryTransfusion MedicineHistocompatibilityImmunologyMolecular PathologyLimited Service LabReproductive Lab(Embryology & Andrology) College of American Pathologists.13 April 202215
Customized Checklists Based on Testing MenuNO SURPRISESCustomized checklists ensureyou know what will beinspected before theinspection based on yourexact testing menu College of American Pathologists.13 April 202216
Incorporating ISO Concepts to StrengthenQualityRequirement DescriptionLaboratory GeneralChecklistExpanding & EnhancingQuality ion of nonconforming eventsRoot Cause Analysis–Focus onCompliance webinar & toolbox*GEN.20318Corrective and preventativeactionGEN.20316QM indicators of qualityTest utilization modules forCAP membersGEN.13806Quality Management SystemQMS–Focus on Compliancewebinar*, QMS examples*, andQMEd course (QMSImplementation Roadmap*)GEN.20326Assess QMS for effectiveness*Resources available in e-LAB Solution Suite College of American Pathologists.13 April 202217
Clarification and Compliance GuidanceExperienced Technical Specialist (Medical Technologist) availablevia phone (800-323-4040) or email (accred@cap.org) Clarify checklist requirements Provide guidance on compliance Educate on best practice College of American Pathologists.13 April 202218
Peer Inspections Reduce RiskTeam inspections cover all aspects of thelaboratory reducing riskPeer inspections reduce risk by encompassingthe entire laboratoryTeams match to size, scope, expertise, andspecialty with laboratory servicesPathologist-led teams bring real worldexperiencesOther accreditors use a “tracer” methodology,narrowing the focus of the inspectionA collegial environment facilitates peer-topeer exchange for mentoring opportunities College of American Pathologists.13 April 202219
A Unique Perspective ThroughReciprocal Inspections 10,000 laboratory professionalsparticipate annually Gain fresh insights and share best practices Identify gaps in your own compliance Learn multiple ways to meet requirements 18 months after your anniversary date College of American Pathologists.13 April 202220
Thorough Inspection Team TrainingOnline TrainingFast Focus on ComplianceCoursework and tools for asuccessful inspectionMini-vignettes providingsupplemental trainingRole specific training forTeam Leader/Team MemberPractical approaches for handlingnew and perplexing topicsEarn CME/CE and SAM creditReal-world scenariosLive Inspector TrainingIn-person events held annuallyOpportunities to engage andinteract with other trainees College of American Pathologists.13 April 202221
What Program Participants Are Saying“Being a CAP inspector has helped me become a bettermedical technologist and supervisor I know how to applythis knowledge to improve quality and enhance patient care.”Kristin Neel, Technical SupervisorTriCore Reference Laboratories“CAP inspections provide a unique opportunity to speak withpeers who are facing the same challenges you are.”Owatha Tatum, PhD, MBA, HLD/CC(ABB)Medical Director, MicroGen Dx“When members of our staff serve on inspection teams, it canactually help us meet our own compliance requirements.”Sheelagh Lindberg, MHA, BSMT(ASCP)Administrative Director, Laboratory, Banner Baywood Medical Center,Banner Heart Hospital/Laboratory Sciences of Arizona College of American Pathologists.13 April 202222
Peer Inspection Options to AccommodateYour NeedsIn-Person InspectionVirtual InspectionAdvance DocumentReview Designated inspection teamvisits facility on site Provides safety for bothinspectors and the laboratory Available for both in-personand virtual inspections Defined day and time forroutine inspection Uses technology to facilitatepeer exchange like digital filetransfer and live video stream Share select documentsbefore the inspection with theinspection team Inspection typically completedin one day Results in an accreditationdecisionTo learn more visit tation/inspector-tools-and-training College of American Pathologists.13 April 202223
Affiliated Inspection OptionsCoordinated Inspection Affiliated laboratories sharing the same anniversary dates Located within 15 miles/30 minutes of each other Same inspection teamStaff Inspection Small laboratories not offering AP or cytopathologyConducted by in-house laboratory scientistsDiverse range of expertiseRepresenting all laboratory disciplinesSystem Inspection All laboratories on the same inspection cycleTwo or more full-service laboratoriesCommon administration and ownershipLocated within a 3-hour drive from a central siteMeet eligibility College of American Pathologists.13 April 202224
A Journey Toward StandardizationAn Integrated Network of Clinical Laboratories atSt. Elizabeth HealthcareEvolve andelevate qualityMultiple sites ofvarying sizeMitigate riskGrow and adaptover time College of American Pathologists.13 April 202225
Complementary Education for ContinuousComplianceFocus onCompliancewebinarsFast Focus onCompliancevignettes Topics to navigate theever-changing rules ofcompliance Specialized content tosupplement inspectortraining Get expert knowledgeon best practices Suitable for laboratorydirectors, managers,and technologists College of American Pathologists. Practical approaches tohandle new andperplexing topics Real-world scenariosfor a clearunderstanding ofrequirementse-LAB SolutionsSuite online portal Obtain FAQ for 75common deficiencies Access customizedcompliance checklists Download templatessuch as Competency,Analytical Validation &Verification Use toolboxes likeIQCP, PT/EQA, RootCause Analysis, Self &Post Inspection13 April 202226
Mitigate Risk Using Real-Time PerformanceDataPerformance Analytics Dashboardweb-based reporting Accreditationo Manage your risk and complianceo Prepare for accreditation inspections Proficiency testingo Perform data analysis to mitigate risk bypatterns & trendso Access evaluations, result forms, andparticipant summary reportso Benchmark your laboratory against your peersand CAP-wide performance Reportingo Focus on relevant details with in-depth analysis College of American Pathologists.13 April 202227
CAP Laboratory Accreditation ProgramsOptional Assessment to Help You Attain Accreditation College of American Pathologists. College of American Pathologists.13 April 202228
Know Where You Stand Before You StartCAP Accreditation Readiness-Assessment (CARA)Gain reassurance by preparing with an on-site evaluationTailoredassessment forkey laboratorypolicies andprocessesConducted byexperiencedCAP entsIdentify areasthat meetstandards orneedimprovementDevelop an action plan using tools from the CAP College of American Pathologists.13 April 202229
Specialty Accreditation ProgramsProgramDescriptionReproductive LaboratoryAccreditation ProgramDesigned for laboratories that perform at least oneembryology-related procedure or perform semen analysis andat least one additional high-complexity test.Forensic Drug TestingAccreditation ProgramDesigned for the unique needs of forensic drug testinglaboratories and intent for laboratories that performconfirmatory testing.Biorepository AccreditationProgramDesigned for biorepositories that collect, process, store, anddistribute biospecimens for research.CAP 15189Accreditation ProgramAccreditation to the ISO standard strengthens the qualitymanagement system throughout the laboratory and all parts ofthe organization that interact with the laboratory, enablingprocess improvement, risk reduction, and improvedoperational efficiencies. Accreditation from the CAP’sLaboratory Accreditation program is a prerequisite. College of American Pathologists.13 April 202230
Begin Your Path to Laboratory Excellence!Let Us Help Start Your Journey TodayEmail Usaccred@cap.orgGet nLearn Morecap.orgBecome CAP Accredited College of American Pathologists.13 April 202231
CAP Laboratory Accreditation ProgramExperienceDiscipline-specific ChecklistsOperational Excellenceheld at the highest standardCollaborative Peer Inspectionsof quality to ensure the bestpatient care College of American Pathologists.Educational Resources13 April 202232
Interactive SessionWe invite you to engage with our speakersby submitting a questionQuestions?Learn more about laboratory ement/accreditation College of American Pathologists.13 April 202233
Accreditation to the ISO standard strengthens the quality management system throughout the laboratory and all parts of the organization that interact with the laboratory, enabling process improvement, risk reduction, and improved operational efficiencies. Accreditation from the CAP's Laboratory Accreditation program is a prerequisite.