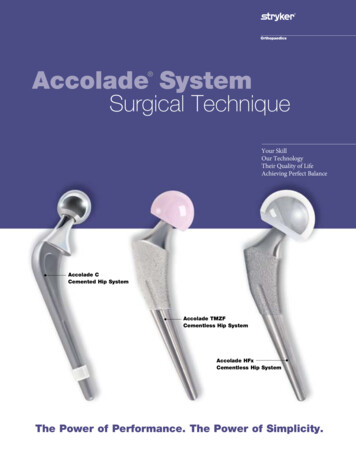
Transcription
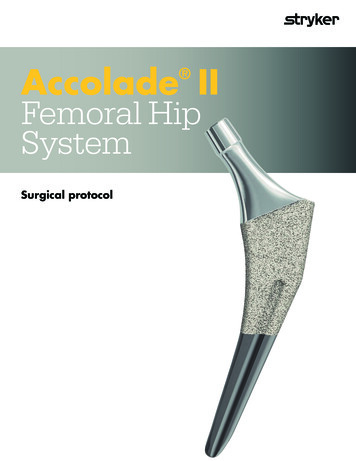
Accolade IIFemoral HipSystem Surgical protocol
Accolade II Femoral Hip System Table of contentsHomeTable of contentsAccolade II Femoral Hip SystemSurgical protocolTable of contentsIndications and contraindications.3Introduction.4Step 1:Preoperative planning.5Step 2:Femoral neck resection.6Step 3:Preparing the femoral canal.7Step 4:Broaching.9Step 5:Trial reduction.11Step 6:Implanting the stem.13Step 7:Final reduction.15Implant information.16Instrumentation.21This publication sets forth detailed validated procedures for using the Accolade II Femoral Hip System. It offers instructions that you should heed, but as withany such technical guide, each surgeon must consider the particular needs of each patient and make appropriate adjustments when and as required.
Accolade II Femoral Hip System Indications and contraindicationsHomeTable of contentsIndications and contraindications and precautionsIndicationsContraindicationsIndications for the U.S. and rest of world:1. Active infection or suspected latent infection in or aboutthe hip joint;The indications for use of the total hip replacementprostheses include:1. noninflammatory degenerative joint disease, includingosteoarthritis and avascular necrosis;2. rheumatoid arthritis;3. correction of functional deformity;4. revision procedures where other treatments or deviceshave failed; and,5. nonunions, femoral neck and trochanteric fracturesof the proximal femur with head involvement that areunmanageable using other techniques.2. Bone stock that is inadequate for support or fixation of theprosthesis;3. Skeletal immaturity4. Any mental or neuromuscular disorder that would createan unacceptable risk of prosthesis instability, prosthesisfixation failure, or complications in postoperative care.5. Obesity. An overweight or obese patient can produce loadson the device that can lead to failure of the fixation of thedevice or to failure of the device itself.Warnings and precautionsAdditional indication specific to use of ACCOLADE II FemoralStems with compatible Howmedica Osteonics ConstrainedLiners:See package insert for warnings, precautions, adverseeffects, information for patients and other essential productinformation.1. When the stem is to be used with compatible HowmedicaOsteonics Constrained Liners, the device is intended foruse in primary or revision patients at high risk of hipdislocation due to a history of prior dislocation, boneloss, soft tissue laxity, neuromuscular disease, or intraoperative instability.Before using Accolade II instrumentation, verify:ACCOLADE II Femoral Stems are intended for cementlessuse only and are intended for total and hemiarth
The Accolade II Hip System is a broach-only system. While . use of an Axial Starter Reamer is needed, use of cylindrical . reamers is not necessary to prepare the femoral canal. The Axial Starter Reamer is used with the T-Handle to open the femoral canal and to aid in determining the orientation