Transcription
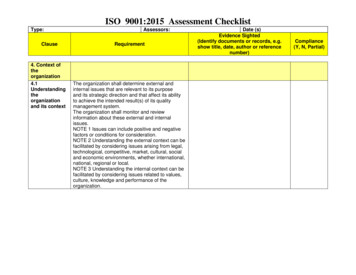
Checklist for AssessmentISO 13485 & MDDRef: xxxxxxChecklist for the assessment based on the standards EN ISO 13485:2016 AC : 2016EN ISO 13485:2016 AC : 2016 associate with EC Directive 93/42 EECIf applicable EC Directive 93/42/EEC Annex II/V/VICompany:Audit date 1. YearAuditor:SignatureNameDieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!Audit date 2. YearAuditor:SignatureNameAudit date 3. YearAuditor:SignatureNameAudit date 4. YearAuditor:SignatureNameAudit date 5. YearAuditor:SignatureName410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.01 / 38
Checklist for AssessmentISO 13485 & MDDRef: xxxxxxExplanations for the application of the DQS assessment check list1.Applicable standards and markingThis assessment checklist is based on the requirements of the standards EN ISO 13485:2016 AC : 2016,MDD 93/42/EEC, Annex II/V/VI and if applicable the German Medical Device Act (MPG).The following references are used to address the requirements of the standards:13485:2016Requirement of the EN ISO 13485:2016 AC:2016MDD/MPG:Questions related to the requirements of the MDD 93/42/EEC (MPG, Germany, resp.).The numbering of the QM-Elements of DIN EN ISO 13485:2016 is used for the chapters.Dieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!2.Use of the Assessment ChecklistThe questions of this checklist are addressed to the auditor, who evaluates and documents the fulfillment of therequirements. The auditor should formulate the questions asked to the company’s representative/s in a differentway, please remember your audit training.The checklist must be used for the documentation of the assessment.The checklist will also be used for 5 years (Certification period for MDD). Therefore 5 columns for the assessment results are given, one for each year. Certification period for 13485 is 3 years. In the 1st year audit every requirements of the standards must be assessed (if applicable). In the 2nd to the 3rd year the audit will be performed on a statistical basis, but in summary within these 2years all requirements must be assessed. The requirements regarding Management responsibility, Internal Audits, Human resources, Production andCorrective & preventive actions must be audited every year.The results of the assessment regarding the documentation and the realization of the QM-System has to bedocumented as follows:1 fulfilled4 not fulfilled3.2 partially fulfilled, acceptablen.a. not applicable3 partially fulfilled, not acceptable0 not auditedAudit protocolThe results of the audit and the objectives/findings must be documented on the protocol “Findings”.In the row „Reference“ the objectives must be referenced to the nomenclature of the questions. Pleaseuse the numbers given in the checklist with 2 digits only (e.g. 4.2 for „Documentation requirements” ofthe ISO 13485). Additional pages, e.g. from the company’s documents should be added to the protocol andnumbered as pages.4.Important Notes / ExemptionsEN ISO 13485:2016 AC:2016 allows exclusions in clauses 6, 7 and 8. To claim compliance with MDD onlycertain exclusions in clause 7 are possible, see below.Because the regulatory requirements of the MDD 93/42 and the German Medical Device Law permit exclusionsof design and development controls (see 7.3), this can be used as a justification for their exclusion from thequality management system.If any requirement(s) in Clause 7 of this International Standard is(are) not applicable due to the nature ofthemedical device(s) for which the quality management system is applied, the organization does not need toinclude such a requirement(s) in its quality management system, but a rational has to be provided [see 4.2.2 a)].The processes required by this International Standard, which are applicable to the medical device(s), but whichare not performed by the organization, are the responsibility of the organization and are accounted for in theorganization’s quality management system [see 4.1 a)].410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.02 / 38
Checklist for AssessmentISO 13485 & MDDRef: xxxxxxThese regulations can provide alternative arrangements that are to be addressed in the quality rGeneral1.1General questions for the certificationDieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!Is the guideline “Regulation for the use of the DQS certificate symbols,the IQ-Net symbol, the DQS documents and the DQS-symbol appliedcorrectly?0.21.2Additional requirements of the 93/42/EEC to establish the procedureMDD/MPGDoes the company keep the correspondence with DQS?MDD/MPGWas the company completely informed about the previous and present(today’s) activities of the auditor(s)? Is the declaration “ Auditors’/Experts’ Independence and Objectivity “ countersigned?MDD/MPGAre the company and the products registered to the legal authorities?MDD/MPGIs there a procedure in place to inform DQS about essential changes ofthe Medical Devices covered by the Quality Management System?(Advice company about the application forms 360.1.11 (Certificationapplication) & 360.1.12 (Product application) and hand it out).4Quality management system4.1General requirementsMDD/MPGIs the MDD 93/42/EEC Annex II/V/VI (and if necessary the MPG/theMPV for Germany) referenced correctly?MDD/MPGIs, if necessary, an authorized representative designated within theEEC?13485:2016Does the organization ensure the control of any outsourced processes,which affect product conformity with requirements and is the control ofsuch outsourced processes identifiable within the QM system?410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.03 / 38
Checklist for AssessmentISO 13485 & MDDRef: xxxxxxEvaluation1.YearDieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!13485:2016Does the organization document a quality management system andmaintain its effectiveness in accordance with the requirements of thisInternational Standard and applicable regulatory requirements?13485:2016Does the organization establish, implement and maintain any requirement, procedure, activity or arrangement required to be documentedby this International Standard or applicable regulatory requirements?13485:2016Does the organization document the role(s) undertaken by the organization under the applicable regulatory requirements?13485:2016Does the organization 13485:2016a) determine the processes needed for the quality management system and the application of these processes throughout the organizationtaking into account the roles undertaken by the organization?13485:2016b) apply a risk based approach to the control of the appropriate processes needed for the quality management system?13485:2016c) determine the sequence and interaction of these processes?13485:2016Does the organization 13485:2016a) determine criteria and methods needed to ensure that both theoperation and control of these processes are effective 13485:2016b) ensure the availability of resources and information necessary tosupport the operation and monitoring of these processes 13485:2016c) implement actions necessary to achieve planned results andmaintain the effectiveness of these processes 13485:2016d) monitor, measure as appropriate, and analyse these processes 13485:2016c) establish and maintain records needed to demonstrate conformance to this International Standard and compliance with applicableregulatory requirements aluation5.Year for each quality management system process?13485:2016The organization manages these quality management system processes in accordance with the requirements of this International Standard and applicable regulatory requirements. Are the following410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.04 / 38
Checklist for AssessmentISO 13485 & MDDRef: YearEvaluation4.YearEvaluation5.Yearchanges to these processes made:Dieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!13485:2016a) evaluated for their impact on the quality management system?13485:2016b) evaluated for their impact on the medical devices produced underthis quality management system?13485:2016c) controlled in accordance with the requirements of this InternationalStandard and applicable regulatory requirements?13485:2016Does the organization monitor and ensure control to any outsourcedprocesses that affects product conformity to requirements?13485:2016Does the organization retain responsibility of conformity to this International Standard and to customer and applicable regulatory requirements for outsourced processes?13485:2016Are the controls proportionate to the risk involved and the ability of theexternal party to meet the requirements in accordance with 7.4?13485:2016Do the controls include written quality agreements?13485:2016Does the organization document procedures for the validation of theapplication of computer software used in the quality management system?13485:2016Are such software applications validated prior to initial use and, asappropriate, after changes to such software or its application?13485:2016Are the specific approach and activities associated with software validation and revalidation proportionate to the risk associated with the useof the software?13485:2016Are records of such activities maintained (see 4.2.5)?4.2Documentation requirements4.2.1General13485:201613485:2016Does the quality management system documentation (see 4.2.4) include:a) documented statements of a quality policy and quality objectives?410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.05 / 38
Checklist for AssessmentISO 13485 & MDDRef: YearEvaluation4.YearEvaluation5.Yearb) a quality manual?13485:2016c) documented procedures and records required by this InternationalStandard?13485:2016d) documents, including records, determined by the organization tobe necessary to ensure the effective planning, operation, and controlof its processes?13485:2016e) other documentation specified by applicable regulatory requirements?13485:2016Dieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!4.2.313485:2016Product documentationAre files available, containing all products specifications and qualityassurance specifications, or is the location of those documents described exactly? ( NB-MED 2.5.1-5)(see also Annex II / V/ VI 93/42/EWG and if necessary DQS-Supportchecklists)13485:2016Is this information available and comprehensible for each type or eachmodel of the medical device? (if necessary also the information forinstallation / maintenance)MDD/MPGAre correct declarations of conformity issued?( EK-MED 3.9 A4)MDD/MPGAre the classifications of the Medical Devices traceable to the MDD?MDD/MPGAre designated harmonized standards, which are applicable to theproduct / procedures defined and available (Is the adherence systematically ensured)? If no harmonized standards are available, is thereproof, which ensures the safety and suitability of the products?MDD/MPGIs it defined and documented for each product how the applicable “Essential Requirements (Annex I)“ are fulfilled and are there proofs tovalidate the statements made?MDD/MPGAre there, for all products/product groups, complete risk analyses defined and documented? (copy the risk analysis to the documents forthe DQS as example; if necessary apply 370.2.13 Checklist Risk Management)MDD/MPGAre all the possible safety risks comprehensibly determined? Are theinitial risks evaluated? Is the evaluation of the risks comprehensible?MDD/MPGDo designated suppliers with activities having a substantial influenceon the quality identified exist?(EK-MED 3.9 B17) (see 360.1.5 BasicData-Organization)410 09e Checkliste for Assessment 13485 MDD.docxVersion: 4.06 / 38
Checklist for AssessmentISO 13485 & MDDRef: xxxxxxEvaluation1.YearDieses Dokument wird bei Ausdrucken oder Ablage an einem anderen als dem ursprünglichen Speicherort ungültig.Jeder Nutzer ist dafür verantwortlich, ausschließlich mit dem jeweils gültigen Ausgabestand des Dokuments zu arbeiten!MDD/MPGAre they listed including their activities and are appropriate certificatesof the subcontractors/ suppliers available?MDD/MPGIf no appropriate certificates for subcontractors/suppliers are available,are they appropriately assessed to fulfill the requirements of the suppliers / subcontractors?MDD/MPGAre contractual declarations/agreements signed with subcontractors/suppliers with defined activities and responsibilities, if they have asubstantial influence on the quality?MDD/MPGDoes the contract with e.g. OEM suppliers contain sufficient descriptions and regulations on notification of the legal authorities and to ensure appropriate information in case of on4.YearEvaluation5.Year( EK-MED 3.9 B16)MDD/MPGIs there a proc
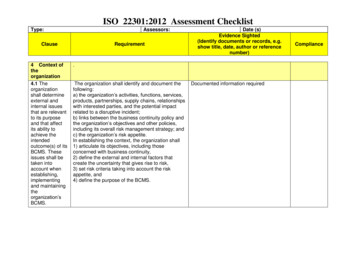
Checklist for the assessment based on the standards EN ISO 13485:2016 AC : 2016 EN ISO 13485:2016 AC : 2016 associate with EC Directive 93/42 EEC If applicable EC Directive 93/42/EEC Annex II/V/VI Company: Audit date 1. Year Auditor: Name Signature Audit date 2. Year Auditor: Name Signature Audit date 3. Year Auditor: Name