Transcription
ASHP REPORTASHP Guidelines on Preventing Medication Errorsin HospitalsMolly Billstein-Leber, Pharm.D.,BCPS, Drug Use Policy and FormularyManagement, Yale New Haven HealthSystem, New Haven, CT.COL Jorge D. Carrillo, Pharm.D.,M.S., M.P.H., BCPS, Department ofPharmacy, Womack Army Medical Center,Fort Bragg, NC.Angela T. Cassano, Pharm.D., BCPS,FASHP, Pharmfusion Consulting, LLC,Midlothian, VA.Kym Moline, Pharm.D., M.S.A.,Medication Safety and ProcessExcellence, Mercy Health Saint Mary’s,Grand Rapids, MI.Jennifer J. Robertson, Pharm.D.,BCPS, Pharmaceutical Department,St. Jude Children’s Research Hospital,Memphis, TN.Address correspondence to BruceHawkins (bhawkins@ashp.org).DOI 10.2146/ajhp170811PurposeThe goal of medication therapy isthe achievement of defined therapeutic outcomes that improve a patient’squality of life while minimizing patient risk.1 There are inherent risks,both known and unknown, associatedwith the use of medications (prescription and nonprescription). This document addresses medication errors,defined asany preventable event that maycause or lead to inappropriate medication use or patient harm while themedication is in the control of thehealthcare professional, patient, orconsumer. Such events may be related to professional practice, healthcare products, procedures, and systems, including prescribing, ordercommunication, product labeling,packaging, and nomenclature, com-occur.9 Blaming healthcare workersinvolved in errors or passively encouraging them to be more careful will notprevent errors since it does not changethe underlying conditions that contributed to the error.The pharmacist should participatein multidisciplinary committees of theorganization and take an active rolein the evaluation and monitoring ofthe medication-use process throughout the hospital or healthcare systemto examine and improve systems toensure that medication processesare safe. Furthermore, health-systempharmacists have the responsibility and expertise to lead collaborative,multidisciplinary efforts to preventmedication-related problems that canresult in patient harm.10The purpose of these guidelines isto provide the pharmacists with practical recommendations and best practices for preventing and mitigatingpatient harm from medication errorsin the health-system setting. Theseguidelines are primarily intended toapply to the acute care setting becauseof the special collaborative processesestablished in this setting (i.e., formulary system, pharmacy and therapeutics committee, widespread useof automation and electronic healthrecords [EHRs], and opportunity forincreased interaction among healthcare providers). However, many of theideas and principles in these guidelines may be applicable to practicesettings outside of the acute care setting, especially in health systems.Medication errors can occur at anypoint of the medication-use system.7For the purposes of these guidelines,the medication-use system is definedin Figure 1.pounding, dispensing, distribution,administration, education, monitoring, and use.2The landmark Institute of Medicine (IOM) report To Err Is Human:Building a Safer Health System, published in 1999, increased the nationalfocus on improvements and the prevention of errors in patient safety.3This report drew attention to the significant problem of medical errorsin the healthcare system, one type ofwhich is medication errors. Other reports published after 1999 have drawnattention to patient safety improvement efforts, including 5-, 10-, and 15year updates after To Err Is Human,4-6as well as the 2007 release of IOM’sPreventing Medication Errors: Quality Chasm Series.7 While the originalIOM report increased awareness ofthe significant risk of medical errors,the pace of change is slow, and there ismore work to be completed.6The outcomes or clinical significance of many medication errors maybe minimal, with few or no consequences that adversely affect a patient. In addition, numerous medication errors go unrecognized and arenot detected or reported. Tragically,however, some medication errors result in serious patient morbidity ormortality.8 Thus, medication errors(including close calls) must not betaken lightly, and risk-reduction strategies and systems should be established to prevent or mitigate patientharm from medication errors.Reason9 stated that humans areimperfect, and errors should be expected. A system-based approachshould be undertaken at institutionsto prevent future errors; this approachstrives to change worker conditionsand build defenses, barriers, and safeguards to prevent errors from occurring or mitigate the harm if errors doAM J HEALTH-SYST PHARM VOLUME 75Planning for safe medicationpracticesSafe medication practices begin NUMBER 19 OCTOBER 1, 2018 1493Downloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020Am J Health-Syst Pharm. 2018; 75:1493-517
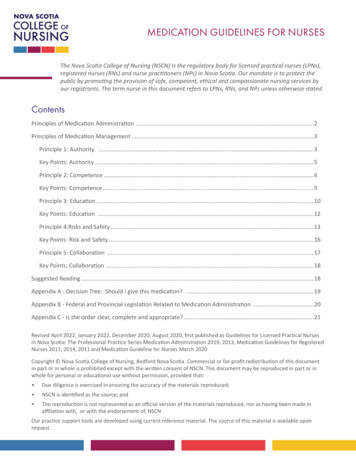
ASHP REPORTPREVENTING MEDICATION ERRORSFigure 1. This diagram is a modification of the Joint Commission’s medication management system, with the addition of2 steps: patient admission and patient discharge. These steps were added to appropriately encompass issues that ariseduring admission and discharge (e.g., medication history and reconciliation errors, patient education barriers).1494AM J HEALTH-SYST PHARM any of these are not well developed,the organization should address themthrough the planning process in orderto meet the continuing goal of ensuring patient safety.A culture of patient safety, basedon the principles of just culture, provides a solid foundation for safe andeffective systems and teamwork. In ajust culture, safety is valued, reportingof safety risks is encouraged withoutpenalization, and the staff, leadership,and board of trustees are held accountable using a clear and transparent process that evaluates the errors.8The evaluation process separatesevents arising from a flawed systemdesign or inadvertent human errorfrom behavioral choices that compromise safety11; there may be conVOLUME 75 NUMBER 19 OCTOBER 1, 2018sequences when unjustifiable risk isknowingly taken by an individual.10 Ajust culture environment should alsoinclude a support system for secondvictims. Second victims are defined ashealthcare providers who are involvedin an unanticipated adverse patientevent, a medical error, or a patientrelated injury and become victimizedin the sense that the provider is traumatized by the event.12,13 Programsshould be established to support thesecond victims and to educate healthcare professionals about the secondvictim effect.14A system for reporting and reviewing errors is an essential componentof a medication safety system; thegoal is to enhance patient safety andprevent patient harm. Errors andDownloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020with placing medication safety as anorganizational and departmental priority, and implementing a system thatwill support these practices. The organization must have a comprehensive program that includes a medication safety leader, key elements inplace to provide the structure for safemedication practices, and a successful strategic plan.10 Key supportingelements include a culture of safetybuilt on principles of just culture thatis supported at all levels of the organization (from the C-suite to the frontline), an event-reporting system, aninterdisciplinary medication safetyteam, a continuous improvement philosophy regarding evaluation of errorsand harm, and strong designs that assess and reduce the risk of errors. If
ASHP REPORTPREVENTING MEDICATION ERRORSand best-practice tools that focuson specific areas, such as automateddispensing cabinets (ADCs) and anticoagulation.22 ASHP also publishespolicy positions and guidelines thatare national best practices nd-Guidelines). A failure modesand effects analysis (FMEA) and a gapanalysis are other methods that can beused to complete a risk assessment.These proactive tools are used to identify the risk of failure before it occurs sothat systems can be designed to minimize risk. Examples of where thesetools can be applied include whenevaluating high-alert medication processes as well as medication–relatedequipment (Appendix B).8,23Reducing the risk of errors.Organizations should prospectivelydesign and implement strategies to reduce certain types of errors in order toprevent patient harm. Areas that mustbe addressed are high-risk populations, high-risk processes, high-alertmedications, and easily confused drugnames, also known as “look-alike/sound-alike” (LASA) medications.Two areas of focus are addressedbelow. High-alert medications aremedications that have an increasedrisk of causing serious patient harmwhen used in error. A hospital-specificlist of high-alert medications may bedeveloped using the ISMP list of highalert drugs in conjunction with thehospital’s patterns of medication useand harm events.24 Risk-reductionstrategies should be implemented thatwill (1) prevent errors, (2) make errorsvisible, and (3) mitigate the harm if anerror occurs.25 Strategies will be successful if they effectively address theunderlying cause of error and impactas many steps of the medication-useprocess as possible; a single riskreduction strategy should not be depended on in most cases. When developing strategies, the literature shouldbe used to identify risk-reductionstrategies that have been proven effective, recommended by experts, orimplemented successfully elsewhere(Appendix A).26AM J HEALTH-SYST PHARM VOLUME 75Examples of safety strategies include but are not limited to Using oral syringes that cannot beconnected to i.v. tubing ports alongwith education on the existence oforal syringes and safe use,Using epidural tubing without ports,Using smart infusion pumps,Using electronic prescribing systemswith clinical decision support,Implementing barcode technologyfor the preparation, dispensing, andadministration of medications,Employing evidence-based standardorder sets and protocols,Standardizing concentrations, diluents, and container sizes,Using scales that only weigh patients in kilograms and documenting weight only in kilograms,Using commercially available products instead of compounding,Dispensing oral and parenteralmedications in the most ready-toadminister form,Using oral measuring devices onlyin metric scale,Performing independent doublechecks on dosing, infusion pumpprogramming, and concentrationswhen appropriate,Utilizing auxiliary labels when appropriate, andImproving readability of labels.Medications that are commonlyconfused due to similarities in name,dosage form, or packaging should alsobe proactively addressed. Medicationsthat are at risk of error can be identified by reviewing local data on errorsand the list of confused drug namespublished by ISMP.27 Strategies shouldbe implemented that address LASAmedication risks. LASA error prevention strategies include differentiation,improved access to information, reminders, limiting access or use, andredundancies.28Strategies for handling LASA medications include Using both brand and genericnames when appropriate,NUMBER 19 OCTOBER 1, 2018 1495Downloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020close calls should be reported andanalyzed (e.g., root cause analysis[RCA]) to identify the causes and develop measures to prevent similar occurrences.5,15,16 Other event detectionmethods, such as trigger tools, chartreview, data from technology, anddirect observation, should be considered to complement error-reportingefforts. There are a number of commercially available software systemsfor online reporting and analysis ofmedication errors.A multidisciplinary medication safety team provides a collaborative and systematic approachto addressing medication safety issues and problems as well as proactively assessing risk.17 In order toensure overall success, a medicationsafety leader, preferably a pharmacist,should lead the medication safety efforts throughout the organization.The “ASHP Statement: Role of theMedication Safety Leader” is an important guide.11 A pharmacist position dedicated to medication safetyshould be developed to ensure thatpharmacists are key safety leaders inthe organization.Lastly, the organization must evaluate and adopt technologies that willhelp reduce the risk of medication errors and help prevent patient harm.18,19Pharmacists must be involved in technology decisions to ensure the safetyand effectiveness of technology thatimpacts the medication-use process.20The application of individual technologies will be discussed in subsequentsections.Risk assessment. The process ofcompleting a medication safety selfassessment will help a health-systemorganization identify medicationsafety risks within its system so thatit can prioritize and plan for improvements. Proactive risk assessmenttools, such as assessments availablefrom the Institute for Safe Medication Practices (ISMP) (www.ismp.org/selfassessments/default.asp), may beused to identify opportunities for improvement through a gap analysis.21ISMP also offers other risk assessment
ASHP REPORT Product packaging is anothersource of look-alike drug errors. Strategies to minimize the risk of error include making items look different bypurchasing products from differentmanufacturers, purchasing differentsize containers, storing drugs in separate areas, and using alerts on theproduct and in the storage area.28The practice of performing independent double checks has beenwidely promoted in healthcare toidentify potential errors before theyreach patients.30 However, misuse andimproper execution of this practicecould jeopardize medication safety.Independent double checks should beselectively applied to certain medications after careful consideration toavoid excessive use and maximize itsintent as an independently performedtask. An independent double checkrequires 2 people and must be conducted independently by the secondperson to reduce bias and increaseeffectiveness. Avoid using independent double checks as a sole-reliancestrategy. Independent double checksshould be implemented in combination with other risk-reduction strategies to reduce the frequency of errors.Pharmacists should be familiarwith which medications are managed via a risk evaluation and mitigation strategy (REMS). REMS isa Food and Drug Administration(FDA)-mandated program that seeksto manage the safe use of a medica-1496AM J HEALTH-SYST PHARM tion with known or potential seriousrisks. The REMS may include a medication guide, patient package insert,communication plan, elements toensure safe use, and an implementation system. Requirements of REMSprograms are not identical amongdifferent medications; thus, it is important for pharmacists to familiarizethemselves with the ASHP ResourceCenter to understand the unique aspects that may exist.31Selection and procurementSelection and procurement ofmedications involve appropriatelyselecting which medications will bestocked in the institution (the formulary) and then safely and effectivelyobtaining the medications from manufacturers and wholesalers. Best practices for decreasing the risk of errorsduring selection and procurementcan generally be divided into 5 categories: (1) formulary assessment andmanagement, (2) standard concentrations, (3) safety-alert monitoring, (4)safe procurement, and (5) medicationshortage management.Formulary assessment andmanagement. A well-designed formulary system will guide cliniciansto prescribe the safest and most costeffective agent for treating a particulardisease state or medical issue.32 Formularies limit the selection of medications available so that cliniciansbecome proficient with the dosing,preparation, and administration practices of a selected number of medications. A streamlined formulary canalso help to standardize the contentof EHRs, pharmacy information systems, and infusion pump settings/medication libraries. Formulariesshould be designed to enhance thesafe use of medications and not simply as a cost-saving measure.The “ASHP Guidelines on the Pharmacy and Therapeutics Committeeand the Formulary System” providedetailed guidance on formularies andmedication evaluation documents(i.e., monographs) and should be consulted for more information.32 In parVOLUME 75 NUMBER 19 OCTOBER 1, 2018ticular, when preparing an evidencebased formulary review document fora medication, a section should be devoted to medication safety assessmentand recommendations. In short, thepharmacist should consider whetherthe medication being reviewed for addition to the formulary has potentialsafety issues, such as a complicatedadmixture or administration process,a similarity in sound or appearance toanother medication (i.e., LASA medication), dosing or duration limitations, a REMS program, admixture oradministration handling precautions,specific requirements on storage orwaste, extravasation management,and significant serious adverse effects that should be monitored. Thisassessment should include a relevantliterature search, including publishedstudies and case reports, manufacturer information, and professional organization and agency websites suchas those of ISMP, FDA (e.g., MedWatchreports), accreditation agencies (e.g.,Joint Commission), Centers for Disease Control and Prevention, NationalInstitute of Occupational Safety andHealth (i.e., occupational safe handling), and the Environmental Protection Agency (e.g., waste precautions).Health-system pharmacists maychoose to develop and use a standardchecklist for medication safety reviewof formulary additions; there are examples on the ASHP Medication-UseSafety Resource Center.33 If the medication is new and limited informationis available, pharmacists need to consider what potential medication safetyissues could arise.When medications with heightened error potential are added to theformulary, strategies to prevent medication errors should be considered.Preferably, these safety enhancements are established and implemented before the initial use of themedication and should be reevaluated as needed.When planning for formulary additions and changes, the medication’sintegration into technology should becarefully coordinated. Dosage forms,Downloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020 Using tall-man lettering, color, orfont to differentiate,29Including the indication for use onorders,Limiting the use of verbal orders,Using read-back processes to minimize errors by spelling the medication name and stating the intendedpurpose,Implementing barcode technologyand/or radio frequency identification (RFID) for the preparation,dispensing, and administration ofmedications, andAvoiding abbreviating drug names ifpossible.PREVENTING MEDICATION ERRORS
ASHP REPORTPREVENTING MEDICATION ERRORSconcentrations, and ordering optionsshould be limited and standardized.Questions to ask when integratingnew formulary medications into technology include the following: Should the routes of administrationavailable for selection be limited?Are tall-man letters needed to distinguish from other medications?Are there significant medication interaction alerts that should be testedfor appropriate firing?Are additional alerts or warningsneeded for laboratory monitoringrequirements, pregnancy contraindications, formulary restrictions, orother issues?Can appropriate and important labresults be displayed during orderentry or verification?Are dose range–checking and smartpump–dosing recommendationsintegrated into the computer systemand pumps? Are they correct?Should an order set be created toease prescribing and monitoringrequirements?Should the item be stored in ADCs?Should the medication be able to beoverridden in ADCs?Are there additional alerts or warnings needed when withdrawing themedication from the ADC?Standard concentrations. Hospitals should standardize and limitthe number of medication concentrations available; indeed, many regulatory agencies require the use ofstandardized concentrations. Standardization may help avoid errorprone calculations, reduce waste,streamline inventory, and facilitatethe use of premixed i.v. solutions.The “rule of 6” should not be used,as this method for calculating concentrations of continuous infusionsled to calculation errors and waste.34When more than 1 concentration isneeded for medications, the institution should use consistent terminology (e.g., double strength, maximum concentration) and consideradditional labeling to distinguishAM J HEALTH-SYST PHARM VOLUME 75through several national distributionchannels.Safe procurement. The pharmacy department must be responsible forall procurement of medications within the organization, including lessobvious patient care areas (e.g., diagnostic imaging, procedural areas).39Medications should not be brought infrom outside sources without collaboration with the pharmacy department(e.g., samples, transfers from otherinstitutions). The “ASHP Guidelines:Minimum Standard for Pharmacies inHospitals” has a section on medication procurement that includes several safety recommendations, includinghow to handle medication samplesand patients’ home medication usein the hospital.39 In short, samplesshould not be used for inpatient treatment and only used for outpatienttreatment with appropriate policiesand procedures in place (e.g., maintenance of records, proper storage).Likewise, a patient’s own medicationsshould only be used after prescriberorder and pharmacist identification.Pharmacists should be actively involved in the evaluation of allmedication device purchasing andreplacement decisions (e.g., pumps)and included in discussions relatedto devices that utilize medicationsfor operational requirements (e.g., dialysis machines). In addition, patientsmay be admitted to hospitals withindwelling pumps, such as pain management or insulin pumps, and policyand procedures must be establishedto ensure safety and continuity of carewith these unique medication deliverysystems.The pharmacy department shouldtake a lead role in coordination of outsourcing services and should consultthe “ASHP Guidelines on Outsourcing Pharmaceutical Services”40 andthe “ASHP Guidelines on OutsourcingSterile Compounding Services.”41Medication shortages management. Hospitals, via the pharmacydepartment, should have a processto communicate medication shortages, including alternatives and sub NUMBER 19 OCTOBER 1, 2018 1497Downloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020 between concentrations (e.g., labelcomments, auxiliary labels). Furthermore, all needed concentrationsshould be available in the pharmacyverification system; conversely, rarelyused or nonformulary concentrationsshould be removed.National standardized concentrations should be used when they areavailable and are therapeutically appropriate. Standardize 4 Safety is anational initiative between ASHP andFDA to develop and implement national standardized concentrations fori.v. and oral liquid medications, bothadult and pediatric. These standardized concentrations, as developed,will be available on the Standardize 4Safety website.35,36Safety-alert monitoring. Medication safety evaluation does not endwhen a medication is added to theformulary. The pharmacy department should continue to monitor theliterature for new medication safetywarnings, in addition to the reviewand analysis of the institution’s medication error reporting data. Analysesand recommendations for handlingsafety alerts that impact the institution should be managed via the medication safety committee or the pharmacy and therapeutics committee.Every institution or hospital system should have an ongoing mechanism to react to medication safetyupdates. ISMP’s website and newsletters are unique resources and providecommunication about medication errors and strategies to prevent their reoccurrence.37 The ISMP website offersmuch of its content free of charge.FDA also provides numerousmethods to keep up-to-date withmedication information. By signingup for the MedWatch E-list (email),clinicians will be notified when MedWatch alerts are released.The National Alert Network (NAN)should be monitored for urgent advisories about serious errors or information requiring immediate attention.These alerts are distributed via ASHPand ISMP.38 NAN alerts are incidentdriven and reach healthcare providers
ASHP REPORTStorageCareful arrangement of medication storage in the pharmacy andthroughout the hospital can help reduce the risk of medication errors. Inthe pharmacy, product arrangementshould minimize unintended selection of the wrong product or dosageform.43,44 Steps to minimize selectionof the wrong product or dosage formin the pharmacy include the following:Ambiguous nomenclature shouldbe avoided. The same drug nomenclature should be used in all databases used throughout the entiremedication-use process (e.g., EHRs,pharmacy information system, infusion pumps, ADCs), using differentiation and screen alerts for medicationsthat may pose a risk for potential errors, such as LASA medications, medications that should not be crushed,and high-alert medications. Whereverpossible, generic names of medications should be used, unless the product is a combination product.Pharmacy inventory should bemanaged to reduce the risk of errorsassociated with drug shortages andexpired medications. A system forrotating stock should be established,and all areas should be monitored forexpired medications and storage atappropriate temperatures. Becausemanaging expired medications canbe challenging, a schedule assigningstaff to regularly inspect and removeexpired medications should be implemented. A process should also be implemented to ensure medications arenot used passed the beyond-use date(i.e., reconstituted bulk bottles).All medications should be storedsecurely; access to secured medication areas should be limited to authorized personnel.44Medications that should notbe stored outside of the pharmacyinclude Use barcode or RFID scanning in thepharmacy to ensure correct products are dispensed,Provide adequate space for eachmedication and strength,Ensure labels on bottles faceforward,Designate separate areas foreach dosage form or route ofadministration,Separate frequently confused pairs,Segregate high-alert medicationsand LASA medications, andUse labeling and alerts whenappropriate.1498AM J HEALTH-SYST PHARM Concentrated electrolytes (i.e.,potassium chloride, 3% sodiumchloride),Concentrated oral opioid solutions,Concentrated insulin U-500,Sterile water in bags,Concentrated epinephrine multidose vials, andNeuromuscular blocking agents.28The use of ADCs on nursing unitscan reduce the frequency of certainmedication errors.25,43,45,46 The ISMPguidance document can be consulted for ensuring safe use of an ADC.47Medications and the quantity thatVOLUME 75 NUMBER 19 OCTOBER 1, 2018will be stocked in the ADC should becarefully selected. Medications shouldbe in ready-to-use, unit dose, or unitof-use containers. Avoid medicationsthat are in bulk supply or those that aremultidose vials. Do not stock medications that require extensive dilutionsor calculations. Barcoding should beused to assist in stocking and restocking the correct medication. Medications should not be removed fromstorage until immediately before administration, and any doses that arenot administered should be returnedto controlled storage promptly. Nursesshould not return medications to theADC, returning them only to the ADCreturn bin. When configuring storagewithin the ADC, the use of individual,locked, locked-lidded compartmentsthat open when the product is selected is preferred for all medications, ifpossible, but at a minimum for highalert medications, reversal agents, anddrugs prone to diversion. If matrixbins are used, each medication andstrength must have a separate bin.Steps should be taken to differentiateLASA medications within the ADC.This may include a more-securedconfiguration of lidded drawers orlocked-lidded drawers to separatethese medications or make the binsmore distinctive. Systematic inventoryaudits should be performed to identify and remove expired and low-usageproducts. The ADC functionality allows for medications to be vendedfrom the machine after medicationorder review and verification by thepharmacist, which is the safest scenario. Functionality of override existsfor emergency situations, which bypasses the pharmacist’s review beforenurse vending of the medication. Theinstitution must define and approvethe specific criteria to allow for medication overrides in emergency situations and specify which specific medication overrides should be allowed.Patient admissionPrescribing errors commonly occur during hospital admission formany reasons, and patients takingDownloaded from 19/1493/5139896 by BIBLIOSAN Remote CILEA CLAS user on 12 March 2020stitution protocols, to prescribersand other clinical staff. The “ASHPGuidelines on Managing Drug Product Shortages in Hospitals and HealthSystems” provides detailed guidance.42The pharmacy department shouldtake a lead role in developing andmanaging a contingency plan in closecollaboration with affected physiciansand health-system committees whenfaced with severe shortages. Just aswhen considering the use of a newmedication, alternative productsshould be examined for possiblemedication safety issues. Oftentimeswith shortages, the medication maycontinue to be available but in adifferent size, dosage form, or concentration, so medication shortageaction plans must examine the implications of these product changesfor frontline staff, dispensing syste
cine (IOM) report To Err Is Human: Building a Safer Health System, pub-lished in 1999, increased the national focus on improvements and the pre-vention of errors in patient safety.3 This report drew attention to the sig-nificant problem of medical errors in the healthcare system, one type of which is medication errors. Other re-