Transcription
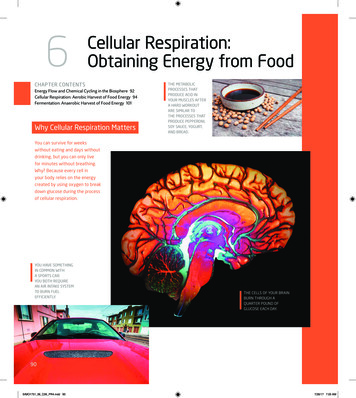
6Cellular Respiration:Obtaining Energy from FoodChapter ContentsEnergy Flow and Chemical Cycling in the Biosphere 92Cellular Respiration: Aerobic Harvest of Food Energy 94Fermentation: Anaerobic Harvest of Food Energy 101Why Cellular Respiration MattersThe metabolicprocesses thatproduce acid inyour muscles aftera hard workoutare similar tothe processes thatproduce pepperoni,soy sauce, yogurt,and bread.You can survive for weekswithout eating and days withoutdrinking, but you can only livefor minutes without breathing.Why? Because every cell inyour body relies on the energycreated by using oxygen to breakdown glucose during the processof cellular respiration.You have somethingin common witha sports car:You both requirean air intake systemto burn fuelefficiently.The cells of your brainburn through aquarter pound ofglucose each day.90SIMO1751 06 C06 PR4.indd 907/28/17 7:03 AM
Chapter ThreadExercise ScienceBiology and Society Getting the Most Out of Your Muscles 91The Process of Science What Causes Muscle Burn? 102Evolution Connection The Importance of Oxygen 103Biology and Society Exercise ScienceGetting the Most Out of Your MusclesSerious athletes train extensively to reach the peak of their physical potential. A key aspect of athletic conditioning involves increasing aerobic capacity, the ability of the heart and lungs to deliveroxygen to body cells. For many endurance athletes, such as long-distance runners or cyclists, the rateat which oxygen is provided to working muscles is the limiting factor in their performance.Why is oxygen so important? Whether you are exercising or just going about your daily tasks, yourmuscles need a continuous supply of energy to perform work. Muscle cells obtain this energy fromthe sugar glucose through a series of chemical reactions that depend upon a constant input of oxygen(O2). Therefore, to keep moving, your body needs a steady supply of O2. When there is enough oxygenreaching your cells to support their energy needs, metabolism is said to be aerobic. As your muscleswork harder, you breathe faster and more deeply to inhale O2. If you continue to pick up the pace,you will approach your aerobic capacity, the maximum rate at which O2 can be taken in and used byyour muscle cells and therefore the most strenuous exercise that your body can maintain aerobically.Exercise scientists can use oxygen-monitoring equipment to precisely determine the maximum possible aerobic output for any given person. Such data allow a well-trained athlete to stay withinaerobic limits, ensuring the maximum possible output—in other words, his or her best effort.If you work even harder and exceed your aerobic capacity, the demand for oxygen in your muscleswill outstrip your body’s ability to deliver it; metabolism then becomes anaerobic. With insufficient O2, your muscle cells switch to an “emergency mode” in which they break down glucose veryinefficiently and produce lactic acid as a by-product. As lactic acid and other wastes accumulate,muscle activity is impaired. Your muscles can work under these conditions for only a few minutesbefore they give out. When this happens (sometimes called “hitting the wall”), your muscles cannot function, and you will likely collapse, unable to even stand.Every living organism depends on processes that provide energy. In fact, we need energy to walk,talk, and think—in short, to stay alive. The human body has trillions of cells, all hard at work, alldemanding fuel continuously. In this chapter, you’ll learn how cells harvest food energy and putit to work with the help of oxygen. Along the way, we’ll consider the implications of how the bodyresponds to exercise.The science of exercise.Endurance athletes mustcarefully monitor theirefforts so that they maintainan aerobic pace over thelong term.91SIMO1751 06 C06 PR4.indd 917/28/17 7:03 AM
6Cellular Respiration:Obtaining Energyfrom FoodCheckpointWhat chemical ingredientsdo plants require from theenvironment to synthesizetheir own food?Energy Flow and Chemical Cyclingin the BiosphereAll life requires energy. In almost all ecosystems on Earth,this energy originates with the sun. During p hotosynthesis,the energy of sunlight is converted to the chemical energyof sugars and other organic molecules (as we’ll discuss inChapter 7). Photosynthesis takes place in the chloroplastsof plants and algae, as well as in some prokaryotes. Allanimals depend on this conversion for food and more.You’re probably wearing clothing made of a product ofphotosynthesis—cotton. Most of our homes are framedwith lumber, which is wood produced by photosynthetictrees. Even textbooks are printed on a material (paper)that can be traced to photosynthesis in plants. But from ananimal’s point of view, photosynthesis is primarily aboutproviding food.minerals Answer: CO2, H2O, and soilProducers and ConsumersPlants and other autotrophs (“self-feeders”) are organismsthat make all their own organic matter—including carbohydrates, lipids, proteins, and nucleic acids—from nutrientsthat are entirely inorganic: carbon dioxide from the air andwater and minerals from the soil. In other words, autotrophsmake their own food; they don’t need to eat to gain energyto power their cellular processes. In contrast, humans andother animals are heterotrophs (“other-feeders”), organisms that cannot make organic molecules from inorganicones. Therefore, we must eat organic material to get ournutrients and provide energy for life’s processes.Most ecosystems depend entirely on photosynthesisfor food. For this reason, biologists refer to plants andother autotrophs as producers. Heterotrophs, in contrast, are consumers because they obtain their food byeating plants or by eating animals that have eaten plants (Figure 6.1). We animals and other heterotrophs dependon autotrophs for organic fuel and for the raw organicmaterials we need to build our cells and tissues.Chemical Cycling betweenPhotosynthesis and CellularRespirationWithin a plant, the chemical ingredients for photosynthesisare carbon dioxide (CO2), a gas that passes from the air intoa plant through tiny pores, and water (H2O), absorbed fromthe soil by the plant’s roots. Inside leaf cells, organellescalled chloroplasts use light energy to rearrange the atomsof these ingredients to produce sugars—most importantlyglucose (C6H12O6)—and other organic molecules (Figure 6.2).You can think of chloroplasts as tiny solar-powered sugarfactories. A by-product of photosynthesis is oxygen gas (O2)that is released through pores into the atmosphere. Figure 6.1 Producerand consumer. A koala (consumer) eating leavesproduced by a photosynthetic plant (producer).92SIMO1751 06 C06 PR4.indd 927/28/17 7:03 AM
and cellular respiration to burn them, while animals perform only cellular respiration.) Plants usually make moreorganic molecules than they need for fuel. This photosynthetic surplus provides material for the plant to grow orcan be stored (as starch in potatoes, for example). Thus,when you consume a carrot, potato, or turnip, you areeating the energy reservoir that plants (if unharvested)would have used to grow the following spring.People have always taken advantage of plants’ photosynthetic abilities by eating them. Morerecently, engineers have managed to tap intothis energy reserve to produce liquid biofuels,primarily ethanol (see Chapter 7 for a discussion of biofuels). But no matter the endproduct, you can trace the energyand raw materials for growthSunlight energyenters ecosystem back to solar-powered photosynthesis.Photosynthesis(in chloroplasts)converts light energyto chemical energyCO2C6H12O6Glucose Carbon dioxide Energy Flow andChemical Cycling in theBiosphereCheckpointWhat is misleading about thefollowing statement? “Plantsperform photosynthesis,whereas animals performcellular respiration.” Answer: It implies that only animals perform cellular respiration,when in fact all life does.A chemical process called cellular respiration usesO2 to convert the energy stored in the chemical bondsof sugars to another source of chemical energy calledATP. Cells expend ATP for almost all their work. In bothplants and animals, the production of ATP during cellular respiration occurs mainly in the organelles called mitochondria (see Figure 4.19).You might notice in Figure 6.2 that energy takes aone-way trip through an ecosystem, entering as sunlightand exiting as heat. Chemicals, in contrast, are recycled.Notice also in Figure 6.2 that the waste products ofcellular respiration are CO2 and H2O—the very sameingredients used as inputs for photosynthesis. Plantsstore chemical energy through photosynthesisand then harvest this energy through cellular respiration. (Note that plantsperform both photosynthesisto produce fuel molecules Figure 6.2 Energy flowand chemical cycling inecosystems. Energy flowsthrough an ecosystem, entering as sunlight and exiting asheat. In contrast, chemicalelements are recycled withinan ecosystem.O2H 2OOxygenWaterFigureWalkthroughMastering Biologygoo.gl/SvLydhCellular respiration(in mitochondria)harvests food energyto produce ATPATPdrives cellular workHeat energy exits ecosystem93SIMO1751 06 C06 PR4.indd 937/28/17 7:03 AM
6Cellular Respiration:Aerobic Harvest of Food EnergyCellular Respiration:Obtaining Energyfrom FoodWe usually use the word respiration to mean breathing.between your blood and the outside air. Oxygen present inAlthough respiration on the organismal level should not bethe air you inhale diffuses across the lining of your lungsconfused with cellular respiration, the two processes areand into your b loodstream. And the CO2 in your bloodclosely related (Figure 6.3). Cellular respiration requiresstream diffuses into your lungs and exits your body whena cell to exchange two gases withyou exhale. Every molecule of CO2 thatCheckpointYouhaveits surroundings. The cell takes inyou exhale was originally formed inAt both the organismal andsomething inoxygen in the form of the gas O2. Itone of the mitochondria of your body’scellular levels, respirationcommon with acells. Internal combustion engines, likegets rid of waste in the form of theinvolves taking in the gassports car: Youand expelling the gasthe ones found in cars, use O2 (throughgas carbon dioxide, or CO2. Res .bothrequireanpiration, or breathing, results inthe air intakes) to break down gasoair intake systemthe exchange of these same gasesline. A cell also requires O2 to breakto burn fueldown its fuel (see Figure 5.2). Cellularefficiently.respiration—a biological version of Figure 6.3 How breathing is related to cellular respiration.internal combustion—is the main wayWhen you inhale, you breathe in O2. The O2 is delivered to yourthatchemicalenergyis harvested from food and convertedcells, where it is used in cellular respiration. Carbon dioxide, a wasteto ATP energy (see Figure 5.6). Cellular respiration is an product of cellular respiration, diffuses from your cells to your bloodand travels to your lungs, where it is exhaled.aerobic process, which is just another way of saying thatit requires oxygen. Putting all this together, we can nowdefine cellular respiration as the aerobic harvesting ofchemical energy from organic fuel molecules.O Answer: O2; CO22An Overview ofCellular RespirationCO2All living organisms depend on transformations ofenergy and matter. We see examples of such transformations throughout the study of life, but few are as important as the conversion of energy in fuel (food molecules)to a form that cells can use directly. Most often, the fuelmolecule used by cells is glucose, a simple sugar (monosaccharide) with the formula C6H12O6 (see Figure 3.6).(Less often, other organic molecules are used to gainenergy.) This equation summarizes the transformationof glucose during cellular respiration:LungsO2O2CO2CO2Many stepsC6H12O6MusclecellsCellularrespiration 6O26CO2 6 H 2O approx. 32ATP heatThe series of arrows in this formula represents the factthat cellular respiration consists of many chemical steps.A specific enzyme catalyzes each reaction in the pathway,more than two dozen reactions in all. In fact, these reactionsconstitute one of the most important metabolic pathwaysfor nearly every eukaryotic cell: those found in plants,fungi, protists, and animals. This pathway provides theenergy these cells need to maintain the functions of life.94SIMO1751 06 C06 PR4.indd 947/28/17 7:03 AM
Figure 6.4 A road mapfor cellular respiration.MitochondriaCellular Respiration:Aerobic Harvestof Food EnergyCheckpointWhich stages of cellularrespiration take place in themitochondria? Which stagetakes place outside themitochondria?electron transport; glycolysishydrogen along with the electrons) that acts as ashuttle carrying high-energy electrons from one areaof the cell to another. The third stage of cellular respiration is electron transport. Electrons capturedfrom food by the NADH formed in the first two stagesare stripped of their energy, a little bit at a time,until they are finally combined with oxygen to formwater. The proteins and other molecules that make up e lectron transport chains are embedded within theinner membrane of the mitochondria. The transportof electrons from NADH to oxygen releases the energyyour cells use to make most of their ATP.The overall equation for cellular respiration showsthat the atoms of the reactant molecules glucose and oxygen are rearranged to form the products carbon dioxideand water. But don’t lose track of why this process occurs:The main function of cellular respiration is to generateATP for cellular work. In fact, the process can producearound 32 ATP molecules for each glucose molecule consumed. Answer: the citric acid cycle andThe many chemical reactions that make up cellularrespiration can be grouped into three main stages: glycolysis, the citric acid cycle, and electron transport.Figure 6.4 is a road map that will help you follow thethree stages of respiration and see where each stageoccurs in your cells. During glycolysis, a moleculeof glucose is split into two molecules of a compoundcalled pyruvic acid. The enzymes for glycolysis arelocated in the cytoplasm. The citric acid cycle (alsocalled the Krebs cycle) completes the breakdown ofglucose all the way to CO2, which is then released asa waste product. The enzymes for the citric acid cycleare dissolved in the fluid within mitochondria. Glycolysis and the citric acid cycle generate a small amountof ATP directly. They generate much more ATP indirectly, by reactions that transfer electrons from fuelmolecules to a molecule called NAD (nicotinamideadenine dinucleotide) that cells make from niacin, aB vitamin. The electron transfer forms a moleculecalled NADH (the H represents the transfer ofCytoplasmCytoplasmAnimal cellPlant cellCytoplasmMitochondrionHigh-energyelectronsvia PCitricAcidCycleATP––ElectronTransport ChainATP95SIMO1751 06 C06 PR4.indd 957/28/17 7:03 AM
6The Three Stages ofCellular RespirationCellular Respiration:Obtaining Energyfrom FoodNow that you have a big-picture view of cellular respiration, let’s examine the process in more detail. A small version of Figure 6.4 will help you keep the overall process ofcellular respiration in plain view as we take a closer lookat its three stages.Stage 1: GlycolysisThe word glycolysisGlycolysismeans “splitting ofsugar” (Figure 6.5),and that’s just whatATPhappens.Duringglycolysis, a six-carbon glucose molecule is broken sport ChainATPhalf, forming two three-carbon molecules. Noticein Figure 6.5 that the initial split requires an energy“investment” of two ATP molecules per glucose.The three-carbon molecules then donate highenergy electrons to NAD , forming NADH.Inaddition to NADH, glycolysis also “banks” four ATP molecules directly when enzymes transfer phosphategroups from fuel molecules to ADP. Glycolysis thusproduces a “profit” of two molecules of ATP permolecule of glucose (two invested, but four banked;this fact will become important during our discussionof fermentation later). What remains of the fracturedglucose at the end of glycolysis are two molecules ofpyruvic acid. The pyruvic acid still holds most of theenergy of glucose, and that energy is harvestedin the second stage of cellular respiration, the citricacid cycle. Figure 6.5 Glycolysis. In glycolysis, a team of enzymes splits glucose,eventually forming two molecules of pyruvic acid. After investing 2 ATP atthe start, glycolysis generates 4 ATP directly. More energy will be harvestedlater from high-energy electrons used to form NADH and from the twomolecules of pyruvic acid.INPUTOUTPUT– –NADHP2PATPPNAD 22 ADP2ATP32 ADPP2 Pyruvic acid1PPP23Glucose2 ADPNAD Key– –NADHCarbon atomP2ATPP Phosphate group– High-energy electronEnergy investment phase:spends 2 ATPEnergy harvest phase:produces 4 ATP96SIMO1751 06 C06 PR4.indd 967/28/17 7:03 AM
INPUT(from glycolysis)Cellular Respiration:Aerobic Harvestof Food EnergyOUTPUT2 Breakdown of the fuel(to citric acid cycle)generates NADH– –NADHNAD CoAPyruvic acid3 Acetic acid attachesAcetic acidto coenzyme Aa carbon as CO2Coenzyme ACO2Stage 2:The Citric Acid CycleCoA escorts the acetic acid into the first reaction of thecitric acid cycle. The CoA is then stripped and recycled.The citric acid cycle finishes extracting the energy ofsugar by dismantling the acetic acid molecules all the waydown to CO2 (Figure 6.7).Acetic acid joins a four-carbonacceptor molecule to form a six-carbon product called citricacid (for which the cycle is named). For every acetic acidmolecule that enters the cycle as fuel,two CO2 molecules eventually exit as a waste product. Along the way,the citric acid cycle harvests energy from the fuel.Someof the energy is used to produce ATP directly. However, thecycle captures much more energy in the form ofNADHanda second, closely related electron carrier calledFADH2.All the carbon atoms that entered the cycle asfuel are accounted for as CO2 exhaust, and the four-carbonacceptor molecule is recycled. We have tracked only oneacetic acid molecule through the citric acid cycle here. Butbecause glycolysis splits glucose in two, the citric acid cycleoccurs twice for each glucose molecule that fuels a cell.CitricDuring glycolysis, one–AcidGlycolysisCycle–molecule of glucose is–Electronsplit into two moleculesTransport Chainof pyruvic acid. ButATPATPbefore pyruvic acid can beATPused by the citric acid cycle,it must be “groomed”—converted to a form the citric acidcycle can use (Figure 6.6).First, each pyruvic acidloses a carbon as CO2. This is the first of this waste product we’ve seen so far in the breakdown of glucose. Theremaining fuel molecules, each with only two carbonsleft, are called acetic acid (the acid that’s in vinegar).Electrons are stripped from these molecules andtransferred to another molecule of NAD , forming moreNADH.Finally, each acetic acid is attached to a molecule called coenzyme A (CoA), an enzyme derived fromthe B vitamin pantothenic acid, to form acetyl CoA. The Figure 6.7The citric acid cycle.INPUT12 CO2PCheckpoint1. Two molecules of whatcompound are producedby glycolysis? Does thismolecule enter the citricacid cycle?2. Pyruvic acid must travelfrom the , whereglycolysis takes place, tothe , where the citricacid cycle takes place.OUTPUTCitricacidAceticacidADP 3Acetyl CoA Answers: 1. Pyruvic acid. No; itis first converted to acetic acid.2. cytoplasm; mitochondria1 Pyruvic acid loses Figure 6.6 The link between glycolysisand the citric acid cycle: the conversionof pyruvic acid to acetyl CoA. Rememberthat one molecule of glucose is split into twomolecules of pyruvic acid. Therefore, theprocess shown here occurs twice for eachstarting glucose molecule.CitricAcidCycleNAD 3FAD2ATP3– –NADH4– –FADH256Acceptormolecule97SIMO1751 06 C06 PR4.indd 977/28/17 7:03 AM
6Cellular Respiration:Obtaining Energyfrom FoodStage 3:Electron TransportCitricDuring cellular respira–AcidGlycolysisCycle–tion, the electrons gath–ElectronTransport Chainered from food moleculesgradually “fall,” losingATPATPATPenergy at each step. In thisway, cellular respirationunlocks chemical energy in small amounts, bit bybit, that cells can put to productive use.Electrons are transferred from glucose in food molecules to NAD . This electron transfer converts NAD toNADH. Then NADH releases two electrons that enter anelectron transport chain, a series of electron carriermolecules. This chain is like a bucket brigade, with eachmolecule passing an electron to the next molecule. Witheach exchange, the electron gives up a bit of energy. Thisdownward cascade releases energy from the electron anduses it to make ATP (Figure 6.8).Let’s take a closer look at the path that electronstake (Figure 6.9). Each link in an electron transportchain is a molecule, usually a protein (shown aspurple circles in Figure 6.9). In a series of reactions,each member of the chain transfers electrons. Witheach transfer, the electrons give up a small amountof energy that can then be used indirectly to generateATP. The first molecule of the chain accepts electronsfrom NADH. Thus, NADH carries electrons from glucose and other fuel molecules and deposits them at the Figure 6.8Cellular respirationillustrated using a hard-hat analogy.Splitting the bonds of a moleculeof glucose provides the energy thatboosts an electron from a low-energystate to a high-energy state.top of an electron transport chain. The molecule at thebottom of the chain finally “drops” the electrons tooxygen. At the same time, oxygen picks up hydrogen,forming water.The overall effect of all this transfer of electronsduring cellular respiration is a “downward” trip forelectrons from glucose to NADH to an electron transport chain to oxygen. During thestepwise release of chemicalenergy in the electron transportchain, our cells make most oftheir ATP. It is actually oxygen, the “electron grabber,”at the end, that makes it allpossible. By pulling electrons down the transportchain from fuel molecules,oxygen functions somewhat like gravity pullingobjects downhill. Becauseoxygen is the final electronacceptor, we cannot survivemore than a few minuteswithout breathing. Viewedthis way, drowning is deadlybecause it deprives cells ofthe final “electron grabbers” (oxygen) needed to drive cellularrespiration. Figure 6.9 The role of oxygen in harvesting food energy. In cellular respiration, electrons “fall” in small steps from food to oxygen, producing water. NADHtransfers electrons from food to an electron transport chain. The attraction ofoxygen to electrons “pulls” the electrons down the chain.Electron releasedfrom NADHATPe–e–e–Electrons from foode–NAD NAD TPe–e–e–Electrontransportchaine–2 e–12O2H 2O2 H Hydrogens, electrons,and oxygen combineto form water98SIMO1751 06 C06 PR4.indd 987/28/17 7:03 AM
downhill, turning giant turbines, and this work can beused to generate electricity. Your mitochondria havestructures that act like turbines. Each of these miniaturemachines, called an ATP synthase, is constructed fromproteins built into the inner mitochondrial membrane,adjacent to the proteins of the electron transport chains.Figure 6.10 shows a simplified view of how the energypreviously stored in NADH and FADH2 can now be usedto generate ATP.NADH andFADH2 transfer electrons to an electron transport chain.The electrontransport chain uses this energy supply to pump H across the inner mitochondrial membrane.Oxygenpulls electrons down the transport chain.The H concentrated on one side of the membrane rushes back“downhill” through an ATP synthase. This action spins acomponent of the ATP synthase, just as water turns theturbines in a dam.The rotation activates parts of thesynthase molecule that attach phosphate groups to ADPmolecules to generate ATP.The poison cyanide produces its deadly effect by binding to one of the protein complexes in the electrontransport chain (marked with a skull-and-crossbonessymbol in Figure 6.10). When bound there, cyanideblocks the passage of electrons to oxygen. This blockage islike clogging the outflow channel of a dam. As a result, noH gradient is generated, and no ATP is made. Cells stopworking, and the organism dies.Cellular Respiration:Aerobic Harvestof Food EnergyCheckpointWhat is the potential energysource that drives ATP production by ATP synthase? Answer: a concentration gradientof H across the inner membrane ofa mitochondrionThe molecules of electron transport chains arebuilt into the inner membranes of mitochondria (see Figure 4.19). Because these membranes are highlyfolded, their large surface area can accommodatethousands of copies of the electron transport chain—a good example of how biological structure fits function. Each chain acts as a chemical pump that usesthe energy released by the “fall” of electrons to movehydrogen ions (H ) across the inner mitochondrial membrane. This pumping causes ions to become more concentrated on one side ofthe membrane thanon the other. Such adifference in concentration stores potential energy, similarto the way water canbe stored behind adam. There is a tendency for hydrogenions to gush back towhere they are lessconcentrated, just asthere is a tendency for water to flow downhill. The innermembrane temporarily “dams” hydrogen ions.The energy of dammed water can be harnessed toperform work. Gates in a dam allow the water to rush Figure 6.10How electron transportdrives ATP synthase esH H ElectroncarrierH H H H H 3– –FADH2ElectronflowFAD1512O2 2 H 6H 2OMitochondrion4NAD H H H 2– –NADHH H ProteincomplexInnermitochondrialmembraneH H H ATPADP PH H H HMatrixElectron transport chainATP synthase99SIMO1751 06 C06 PR4.indd 997/28/17 7:03 AM
6Cellular Respiration:Obtaining Energyfrom FoodThe Results ofCellular Respiration Figure 6.12 Energy fromfood. The monomers fromcarbohydrates (polysaccharidesand sugars), fats, and proteinscan all serve as fuel for cellularrespiration.When taking cellular respiration apart to see how allthe molecular nuts and bolts of its metabolic machinery work, it’s easy to lose sight of its overall function: toFoodgenerate about 32 molecules of ATP per molecule of glucose (the actual number can vary by a few, dependingon the organism and molecules involved). Figure 6.11will help you keep track of the ATP molecules generFatsProteinsCarbohydratesated. As we discussed, glycolysis and the citric acidcycle each contribute 2 ATP by directly making it. Therest of the ATP molecules are produced by ATP synthase, powered by the “fall” of electrons from food toSugarsGlycerolFatty acidsAmino acidsoxygen. The electrons are carried from the organic fuelto electron transport chains by NADH and FADH2. Eachelectron pair “dropped” down a transport chain from–NADH or FADH2 can power the synthesis of a few ATP.Citric–AcetylYou can visualize the process like this: Energy flows fromGlycolysisAcidCoA–Cycleglucose to carrier molecules and ultimately to ATP.ElectronTransport ChainWe have seen that glucose can provide the energyto make the ATP our cells use for all their work. All ofthe energy-consuming activities of your body— ATPmoving your muscles, maintaining your heartThe cells ofbeat and temperature, and even the thinkingyour brainthat goes on within your brain—can be tracedBut even though we have concentrated on glucose asburn through aback to ATP and, before that, the glucose thatthe fuel that is broken down during cellular respiration,quarter poundwas used to make it. The importance of glucoserespiration is a versatile metabolic furnace that can “burn”of glucoseis underscored by the severity of diseases inmany other kinds of food molecules. Figure 6.12 diagramseach day.which glucose balance is disturbed. Diabetes,some metabolic routes for the use of carbohydrates, fats,which affects more than 20 million Americans, is causedand proteins as fuel for cellular respiration. Taken together,by an inability to properly regulate glucose levels in theall of these food molecules make up your hormoneinsulin.Ifleftmetabolism. The interplay between these pathwaysWhich stage of cellular respiration produces theuntreated, a glucose imbalance can lead to a variety ofprovides a clear example of the theme of system inter majority of ATP?problems, including cardiovascular disease, coma, andactions; in this case, all of these interactions contributeeven death.to maintaining a balanced metabolism. Answer: electron transport Figure 6.11 A summaryof ATP yield during Glucose2Pyruvicacid2ATPby tronTransport Chain2ATPAbout28 ATPby directsynthesisMaximumperglucose:About32 ATPby ATPsynthase100SIMO1751 06 C06 PR4.indd 1007/28/17 7:03 AM
Fermentation:Anaerobic Harvest of Food EnergyFermentation inHuman Muscle CellsYou know by now that as your muscles work, they requirea constant supply of ATP, which is generated by cellularrespiration. As long as your blood provides your musclecells with enough O2 to keep electrons “falling” downtransport chains in mitochondria, your muscles will workaerobically.But under strenuous conditions, your muscles canspend ATP faster than your bloodstream can deliverO2 ; when this happens, your muscle cells begin towork anaerobically. After functioning anaerobicallyfor about 15 seconds, muscle cells will begin to generate ATP by the process of fermentation. Fermentationrelies on glycolysis, the first stage of cellular respiration. Glycolysis does not require O2 but does producetwo ATP molecules for each glucose molecule broken down to pyruvic acid. That isn’t very efficientcompared with the 32 or so ATP molecules each glucosemolecule generates during cellular respiration, but itcan energize muscles for a short burst of activity.
between your blood and the outside air. Oxygen present in the air you inhale diffuses across the lining of your lungs and into your bloodstream. And the CO 2 in your blood-stream diffuses into your lungs and exits your body when you exhale. Every molecule of CO 2 that you exhale was originally formed in one of the mitochondria of your body's .