Transcription
10Early Experiences Can Alter GeneExpression and Affect Long-TermDevelopmentworking paper10
memberscontributing membersJack P. Shonkoff, M.D., ChairJulius B. Richmond FAMRI Professor of Child Health andDevelopment, Harvard School of Public Health and HarvardGraduate School of Education; Professor of Pediatrics,Harvard Medical School and Children’s Hospital Boston;Director, Center on the Developing Child, Harvard Universitysusan nall balesPresident, FrameWorks InstitutePat Levitt, Ph.D., Science DirectorDirector, Zilkha Neurogenetic Institute; Provost Professor ofNeuroscience, Psychiatry & Pharmacy; Chair, Department ofCell and Neurobiology, Keck School of Medicine, University ofSouthern CaliforniaW. Thomas Boyce, M.D.Sunny Hill Health Centre/BC Leadership Chair in ChildDevelopment; Professor, Graduate Studies and Medicine,University of British Columbia, VancouverJudy Cameron, Ph.D.Professor of Psychiatry, University of PittsburghGreg J. Duncan, Ph.D.Distinguished Professor, Department of Education,University of California, IrvineNathan A. Fox, Ph.D.Distinguished University Professor; Director, ChildDevelopment Laboratory, University of Maryland College ParkMegan Gunnar, Ph.D.Regents Professor and Distinguished McKnight UniversityProfessor, Institute of Child Development, Universityof MinnesotaLinda C. Mayes, M.D.Arnold Gesell Professor of Child Psychiatry, Pediatrics, andPsychology, Yale Child Study Center;Special Advisor to the Dean, Yale School of MedicineBruce S. McEwen, Ph.D.Alfred E. Mirsky Professor; Head, Harold and MargaretMilliken Hatch Laboratory of Neuroendocrinology,The Rockefeller Universityphilip a. fisher, ph.D.Professor of Psychology, University of OregonSenior Research Scientist, Oregon Social LearningCenter & Center for Research to Practicewilliam greenough, ph.D.Swanlund Professor of Psychology, Psychiatry, and Cell andDevelopmental Biology; Director, Center for Advanced Studyat University of Illinois, Urbana-Champaigneric knudsen, ph.D.Edward C. and Amy H. Sewall Professor of Neurobiology,Stanford University School of MedicineDeborah phillips, ph.D.Professor of Psychology and Associated Faculty, Public PolicyInstitute; Co-Director, Research Center on Children in the U.S.,Georgetown Universityarthur J. rolnick, ph.D.Senior Vice President and Director of Research, FederalReserve Bank of Minneapolispartnersthe frameworks institutethe national governors association center for best practicesthe national conference of state legislaturessponsorsthe birth to five policy alliancethe buffett early childhood fundPalix foundationCharles A. Nelson III, Ph.D.Richard David Scott Chair in Pediatric DevelopmentalMedicine Research, Children’s Hospital Boston; Professor ofPediatrics and Neuroscience, Harvard Medical SchoolRoss Thompson, Ph.D.Professor of Psychology, University of California, DavisAbout the AuthorsThe National Scientific Council on the Developing Child, housed at the Center on the Developing Child at Harvard University, is amulti- disciplinary collaboration designed to bring the science of early childhood and early brain development to bear on publicdecision- making. Established in 2003, the Council is committed to an evidence-based approach to building broad-based public will thattranscends political partisanship and recognizes the complementary responsibilities of family, community, workplace, and government topromote the well-being of all young children. For more information, go to www.developingchild.net.Please note: The content of this paper is the sole responsibility of the Council and does not necessarily represent the opinions ofthe funders or partners.Suggested citation: National Scientific Council on the Developing Child (2010). Early Experiences Can Alter Gene Expression and AffectLong-Term Development: Working Paper No. 10. http://www.developingchild.net May 2010, National Scientific Council on the Developing Child, Center on the Developing Child at Harvard Universityfirst printing: May 2010
The Issuenew scientific research shows that environmental influences can actually affectwhether and how genes are expressed. Thus, the old ideas that genes are “set in stone” or that theyalone determine development have been disproven. In fact, scientists have discovered that early experiences can determine how genes are turned on and off and even whether some are expressed atall.1,2,3 Therefore, the experiences children have early in life—and the environments in which theyhave them—shape their developing brain architecture and strongly affect whether they grow up tobe healthy, productive members of society. This growing scientific evidence supports the need forsociety to re-examine the way it thinks about the circumstances and experiences to which youngchildren are exposed.The approximately 23,000 genes that childreninherit from their parents form what is called the“structural genome.” Scientists liken the structural genome to the hardware of a computer—both determine the boundaries of what’s possible, but neither works without an operating system to tell it what to do. In the genome, that operating system is called the epigenome.4 Like thesoftware in an operating system, the epigenomedetermines which functions the genetic “hardware” does and does not perform.5 This systemis built over time as positive experiences, such asexposure to rich learning opportunities, or negative influences, such as environmental toxinsor stressful life circumstances, leave a chemical“signature” on the genes. These signatures canbe temporary or permanent, and both types affect how easily the genes are switched on or off.For example, even though identical twins havethe same structural genomes, their different experiences result in different epigenomes.6 Thesediffering experiences leave signatures on theepigenome that cause some genes to be expresseddifferently. This explains why genetically identical twins, though similar in many ways, can exhibit different behaviors, skills, health, and achievement in both school and, later, in the workplace.The field of epigenetics is relatively newand at the cutting-edge of the biological sciences. To date, scientists have found thattemporary epigenetic chemical modificationscontrol when and where most of our genes areturned on and off. This, however, is not the entire story. Certain experiences can also causeenduring epigenetic modifications in hundreds of genes that have already beenidentified, and the list is growing.7,8 Increasingwww.developingchild.NETevidence shows that experience-driven, chemical modifications of these latter genes appearto play particularly key roles in brain and behavioral development. This new knowledgehas motivated scientists to look more closelyat the factors that shape the epigenome and tostudy whether interventions can reverse thesemodifications when negative changes occur.Nutritional status, exposure to toxins anddrugs, and the experiences of interacting withvaried environments can all modify an individual’s epigenome.9 Epigenetic instructions that change how and when certain genesare turned on or off can cause temporary orLike the software in a computer’s operating system,the epigenome determines which functions thegenetic “hardware” does and does not perform.enduring health problems. Moreover, researchin both animals and humans shows that someepigenetic changes that occur in the fetus during pregnancy can be passed on to later generations, affecting the health and welfare of children, grandchildren, and their descendents.10,11,12For example, turning on genes that increase cellgrowth, while at the same time switching offgenes that suppress cell growth, has been shownto cause cancer.13,14 Repetitive, highly stressful experiences can cause epigenetic changesthat damage the systems that manage one’s response to adversity later in life.2,3,15 On the otherhand, supportive environments and rich learning experiences generate positive epigeneticEarly Experiences Can Alter Gene Expression and Affect Long-Term Development 1
National scientific council on the developing childsignatures that activate genetic potential.16 Inthis second case, the stimulation that occursin the brain through active use of learning andmemory circuits can result in epigenetic changes that establish a foundation for more effectivelearning capacities in the future.17,18As we get older, new experiences can continue to change our epigenome. However, sciencetells us that the chemical signatures imprintedon our genes during fetal and infant development can have significant influences on brainarchitecture that last a lifetime. Stated simply, the discovery of the epigenome providesan explanation, at the molecular level, for whyand how early positive and negative experiencescan have lifelong impacts.2,3,19,20Policymakers can use this knowledge toinform decisions about the allocation of resources for interventions that affect the lifecircumstances of young children—knowingthat effective interventions can literally alter how children’s genes work and, thereby,have long-lasting effects on their mental andphysical health, learning, and behavior. Inthis respect, the epigenome is the crucial linkbetween the external environments that shapeour experiences and the genes that guide ourdevelopment.What Science Tells Usover the past 50 years, extensive researchhas demonstrated that the healthy development of all organs, including the brain, dependson how much and when certain genes are expressed. When scientists say that genes are “expressed,” they are referring to whether they areturned on or off—essentially whether and whengenes are activated to do certain tasks. Researchhas shown that there are many non-inheritedenvironmental factors and experiences thathave the power to chemically mark genes andcontrol their functions. These influences createa new genetic landscape, which scientists callthe epigenome. Some of these experiences leadto chemical modifications that change the expression of genes temporarily, while increasingnumbers have been discovered that leave chemical signatures that result in an enduring changein gene expression.critical periods of modification, while other genesare open to alterations throughout life.2,3,22,23,24,25Epigenetic modification typically occurs incells that comprise organ systems, thereby influencing how these structures develop and function. Experiences that change the epigenomeearly in life, when the specialized cells of organssuch as the brain, heart, or kidneys are first developing, can have a powerful impact on physical and mental health for a lifetime.26 We arealso learning from new scientific discoveries inboth animals and humans that environmentalfactors, such as certain drugs or the nutritionalstatus of the mother, have the potential to causeepigenetic changes to genes in egg or sperm cellsin the fetus. When such changes occur, this newchemical signature of the DNA is enduring andcan be inherited by future generations.27,28,29Early prenatal or postnatal experiences and exposures influence long-term outcomes by chemically altering the structure of genes. Known asThe brain is particularly responsive to experiences and environments during early development, which influences how well or poorly itsarchitecture matures and functions. We knowepigenetic modification (from the Greek rootepi, meaning upon or over), these chemical signatures are written on top of the gene withoutactually altering the genetic code itself.21 Instead,the signatures attract or repel other chemicalsthat help the genes produce the proteins thatare the building blocks our brains and bodies need to develop. Research tells us that somegenes can only be modified epigenetically during certain periods of development, defined asfrom extensive research that the physiologicalactivity created by experience is powerful inshaping brain architecture and actually changesthe chemistry that encodes the genes in braincells.30 Put simply, the brain adapts to the experiences it has. Certain types of adaptations result in healthy systems, such as effective learningand memory, and other adaptations lead to thedevelopment of unhealthy systems, such as setting a stress response activation level too high2 Early Experiences Can Alter Gene Expression and Affect Long-Term Developmentwww.developingchild.NET
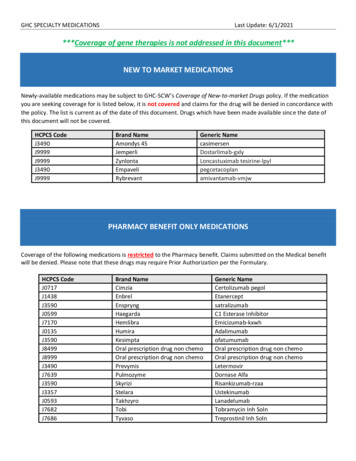
What science Tells Usor too low. The physiological activity caused bypositive mastery experiences can lead to epigenetic changes that control the expression ofgenes in brain cells that are essential for successful learning.17,18,31 In a parallel fashion, exposureto damaging levels of stress early in life can leadto long-lasting epigenetic changes in brain cellsthat direct how our bodies respond to adversitythroughout the lifespan.32 In short, early experiences cause epigenetic adaptations in the brainthat influence whether, when, and how genesbuild the capacity for future skills to develop.Modification of the epigenome caused by stressduring fetal and child development affects howwell or poorly we respond to stress as adultsand can result in increased risk of adult disease.Some of our genes provide instructions for howour bodies respond to stress, and research hasshown that these genes are clearly subject toepigenetic modification. For example, researchin animals has shown that stressful experiencesto which the pregnant mother is exposed, or towhich the offspring is exposed soon after birth,can produce epigenetic changes that chemicallymodify the receptor in the brain that controlsthe stress hormone cortisol and, therefore, determines the body’s response to threat (thefight-or-flight response).19,33,34 Healthy stressresponses are characterized by an elevation inblood cortisol followed by a return to baselineto avoid a highly activated state for a prolongedperiod of time. If young children or pregnantmothers experience toxic stress—as a resultof serious adversity (such as chronic neglect,abuse, or exposure to violence) in the absenceof protective relationships—persistent epigenetic changes can result.32 These modificationshave been shown to cause prolonged stress responses, which can be likened to revving a carengine for long periods of time. Excessive stresshas been correlated with changes in brain architecture and chemistry as well as animal behaviors that resemble anxiety and depression inHow Early Experiences Alter Gene Expression and Shape Development1 EXTERNAL EXPERIENCES(e.g., stress, nutrition, toxins)spark signals between neurons3 GENE REGULATORY PROTEINSattract or repel enzymes thatadd or remove epigenetic markers2 NEURAL SIGNALS launchproduction of gene regulatoryproteins inside cell4 E PIGENETIC “MARKERS” controlwhere and how much protein is madeby a gene, effectively turning a gene“on” or “off,” thereby shaping howbrains and bodies developILLUSTRATION BY BETSY HAYESgene – a specificsegment of aDNA strandDNA strands encircle histones that determinewhether or not the gene is “readable” by the cellNeuron (brain cell)chromosome – can passon genes to next generationwww.developingchild.NETEarly Experiences Can Alter Gene Expression and Affect Long-Term Development 3
National scientific council on the developing childhumans.35,36,37,38,39,40 Human studies have foundconnections between highly stressful experiences in children and increased risk for latermental illnesses, including generalized anxietydisorder and major depressive disorder.41,42,43Atypical stress responses over a lifetime can alsoresult in increased risk for physical ailments,such as asthma, hypertension, heart disease anddiabetes.29,32,41,42,43,44,45,46,47,48In addition to adverse experiences, a wide variety of chemicals, nutrients, and drugs are alsocapable of modifying the epigenome for longlasting effects on gene expression. Epigeneticmodification caused by exposure to toxic substances can either turn genes off or on, and bothconditions have been linked to increased risk formental and physical illnesses. Certain dietarysupplements (such as excessive amounts of folic acid, choline, or vitamin B12) and chemicals(such as bisphenol A, or BPA, which is foundin some plasticware) can turn genes off.1,8,49,50Some genes that can be turned on by heavy metals, such as cadmium, nickel, and lead, have beenlinked to the cellular over-activity that resultsin an increased risk for certain kinds of cancer.28,51,52 Many organs, including the brain, aremost vulnerable to the influence of these substances on gene expression during the period offetal and infant development, when basic organsystems are being built. These resulting differences in gene expression, in turn, can lead tofundamental changes in brain architecture andthe biological systems that govern how well wefunction later in life.Recent research demonstrates that even after theepigenome has been modified, there may be waysto alter it again that actually can reverse negativechanges and restore functioning. Experimentsin animals have shown that certain types of epigenetic modifications that were thought to bepermanent can be reversed under certain conditions.35,53 Most recently, researchers found thatstressful experiences during early postnatal development resulted in epigenetic modificationthat caused exaggerated stress responses in adultanimals, and that subsequent drug treatment ofthe adults could eliminate these adverse DNAchanges. In this case, reversing the chemicalmodification resulted in increased expression ofgenes that control the stress response, and whenexposed to subsequent stress, the treated adultanimals had a normal response (that is, the adverse effect of the early postnatal experiencehad, indeed, been reversed). We now know thatthe same types of epigenetic chemical modifications can occur in adult humans who enduredextreme stress as children, such as from physical abuse.19 This possibility of reversibility isgenerating a flurry of research activity, becauseit has direct implications for developing newinterventions for physical and mental illnessesthat we now know are due in part to epigeneticmodification. As promising as this work appearsto be, further research is needed to determine ifand how the reversal of epigenetic modificationscan be achieved in humans.Correcting Popular Misrepresentations of Scienceuntil recently, the influences of geneswere thought to be set, and the effects of children’s experiences and environments on brainarchitecture and long-term physical and mentalhealth outcomes remained a mystery. That lackof understanding led to several misleading conclusions about the degree to which negative andpositive environmental factors and experiencescan affect the developing fetus and young child.The following misconceptions are particularlyimportant to set straight.4 Early Experiences Can Alter Gene Expression and Affect Long-Term DevelopmentContrary to popular belief, the genes inheritedfrom one’s parents do not set a child’s futuredevelopment in stone. Variations in DNA se-quences between individuals certainly influencethe way in which genes are expressed and howthe proteins encoded by those genes will function. But that is only part of the story—the environment in which one develops, before andsoon after birth, provides powerful experiencesthat chemically modify certain genes which,in turn, define how much and when they areexpressed. Thus, while genetic factors exertwww.developingchild.NET
correcting popular misrepresentations of sciencepotent influences, environmental factors havethe ability to alter family inheritance.Although frequently misunderstood, adversefetal and early childhood experiences can—anddo—lead to physical and chemical changes inthe brain that can last a lifetime. Injurious ex-periences, such as malnutrition, exposure tochemical toxins or drugs, and toxic stress beforebirth or in early childhood are not “forgotten,”but rather are built into the architecture of thedeveloping brain through the epigenome. The“biological memories” associated with theseepigenetic changes can affect multiple organsystems and increase the risk not only for poorphysical and mental health outcomes but alsofor impairments in future learning capacity andbehavior.Despite some marketing claims to the contrary,the ability of so-called enrichment programs toenhance otherwise healthy brain developmentis not known. While parents and policymak-ers might hope that playing Mozart recordingsto newborns will produce epigenetic changesthat enhance cognitive development, thereis absolutely no scientific evidence that suchexposure will shape the epigenome or enhancebrain function. What research has shown isthat specific epigenetic modifications do occur in brain cells as cognitive skills like learning and memory develop and that repeatedactivation of brain circuits dedicated to learning and memory through interaction with theenvironment, such as reciprocal “serve and return” interaction with adults,54 facilitates thesepositive epigenetic modifications. We also knowAdverse fetal and early childhood experiencescan—and do—lead to physical and chemicalchanges in the brain that can last a lifetime.that sound maternal and fetal nutrition, combined with positive social-emotional supportof children through their family and community environments, will reduce the likelihood ofnegative epigenetic modifications that increasethe risk of later physical and mental healthimpairments.The Science-Policy Gapthe fact that the genome is vulnerable tomodification by toxic stress, nutritional problems, and other negative influences underscoresthe importance of providing supportive andnurturing experiences for young children inthe earliest years, when brain development ismost rapid. From a policy perspective, it is insociety’s interest to strengthen the foundationsof healthy brain architecture in all young children to maximize the return on future investments in education, health, and workforce development. In this context, the epigenome is thechemical signature that explains how early lifeexperiences become embedded in the circuitryof the developing brain and are associated withlifelong consequences. Research now shows thatinteraction between adverse environments andthe genes we inherit—through the epigenome—can increase the risk for long-term negativemental and physical health outcomes. Nevertheless, many policy decisions do not yet reflectwww.developingchild.NETthis growing knowledge, which logically calls forreducing the exposure of pregnant women andyoung children to environments and experiences that can have significant negative effects onthe epigenome (and therefore powerful, indirectinfluences on genes). This gap between what weknow and what we do is illustrated by the following four examples:Child welfare. Because threatening or harmfulenvironments can produce epigenetic modifications that affect a child adversely for a lifetime, the management of child abuse or neglectcases by child protective services is extremelytime-sensitive. This sense of urgency is particularly important with respect to decisions aboutcustody arrangements that involve non-familyplacements. The biology of adversity also tellsus that assessment and planning for the mental health and broad developmental needs ofvulnerable children—with the assurance ofEarly Experiences Can Alter Gene Expression and Affect Long-Term Development 5
National scientific council on the developing childnurturing, protective, and stable relationshipsas the highest priority—require attention in thechild welfare system comparable to the conventional focus placed primarily on physical safety.Mandated maternal employment and public assistance. Whether low-income mothers withvery young children should be required to workin order to be eligible for public assistance is apolitical decision. Policies informed by scientific knowledge about early brain developmentand epigenetics, however, would link mandatedmaternal employment to a parallel investmentin high-quality early care and education programs for affected children. When policies viewchild care simply as a custodial service whoseprimary purpose is to facilitate maternal workoutside the home, they reflect a lack of understanding of extensive scientific evidence abouthow the developing architecture of the brain isshaped by epigenetic influences associated withthe quality of adult-child interactions in theearly childhood years. In contrast, policies thatview high-quality child care and early educationprograms as strategic interventions to improvethe life prospects of children whose parents havelimited education and low income are morelikely to increase the prosperity of communitiesacross generations.Prenatal and newborn health care. The fetalperiod is a highly active time for organ development and epigenetic modification, yet investment in prenatal services remains uneven.Policies that assure the availability, accessibility,and affordability of individualized support andmonitoring of all pregnancies create a safety netthat prevents harm and detects problems at apoint when appropriate interventions can reduce the negative consequences of toxic stressand other adverse environmental exposures.Support for new parents. The United States isone of very few developed nations that does notprovide some amount of paid family leave forall parents after the birth or adoption of a baby.For parents of newborns who do not have theeconomic resources to make ends meet in theabsence of paid employment, the supportiverelationships that promote positive epigeneticchanges and help very young children to manage stress can be compromised by a prematurereturn to work and the inability to secure highquality care for a young infant. Family leave isone way of helping parents build these criticalrelationships, but other policies that supportparents during this important transition timecan also have important, short-term effects onthe quality of family life as well as long-term impacts that bring high returns to all of society.Implications for Policy and Programsbecause early experiences can alter theepigenome and influence developing brain architecture, policies affecting the life circumstances of pregnant women and young childrencan have enormous implications for all of society. The varied effects of environments on theepigenome are evident from the time of earlyembryonic development and extend into theearly childhood years. Science tells us that children can be helped to reach their full potentialthrough both appropriate experiences in theearliest years and the reduction of sources oftoxic stress that can alter the epigenome and increase the risk of long-term problems in physical and mental health. Thus, public policies thatharness the basic principles of neuroscienceand epigenetics to address the needs of young6 Early Experiences Can Alter Gene Expression and Affect Long-Term Developmentchildren are likely to also generate long-termbenefits, such as healthier communities and amore prosperous society.The documented effects of toxic stress on negative epigenetic adaptations demonstrate theurgent need to alleviate sources of significantadversity as early as possible in the lives of children who live in threatening environments. Inorder to be maximally effective, programs andservices targeting the precipitants of excessivestress—such as child abuse and neglect, violentneighborhoods and families, and caregiver mental illness—must have prompt access to specificexpertise in the target areas in order to movequickly to protect young children from epigenetic changes that can lead to lifelong problemswww.developingchild.NET
implications for policy and programsin learning, behavior, and health. Because theclock is always ticking when the basic architecture of the brain is developing, informedpolicymakers understand that a delayed response to the needs of young children who areexperiencing significant adversity jeopardizestheir individual well-being as well as the broaderhuman capital needs of society.Epigenetic changes caused by the exposure ofpregnant women, infants, and toddlers to environmental toxins, prescription drugs, alcohol,and illicit substances require an urgent look atwhat safeguards can be implemented to preventsuch exposures. Lead paint laws are a good ex-ample of public policy that has been successfulin reducing the harmful consequences of environmental toxins. Less aggressive policies withrespect to mercury and organophosphate insecticides, on the other hand, are just two examplesof many missed opportunities to mitigate thewell-documented, adverse effects of environmental hazards on pregnant women and youngchildren. The serious and continuing impact ofprenatal exposure to alcohol and a wide varietyof chemical substances (including prescriptiondrugs) on child health and development callsfor a more vigorous approach to environmental policies and public education. In view of thewell-established scientific fact that embryonic,fetal, and early childhood brain development isconsiderably more susceptible to damage fromneurotoxins than the mature brain of an adult,the establishment of safe levels of exposure totoxic substances should be based on scientificdata that recognize the critical link between vulnerability and age, and that focus primarily onthe best information available for the youngestchildren.55Because prenatal and early postnatal experiences can affect long-term outcomes throughepigenetic influences, the provision of highquality health services and nutritional supportfor all pregnant women, infants, and toddlerswould be likely to reduce preventable diseasesacross the lifespan—as well as the costly treatments for them. A range of currently availableprograms and systems has been established tomeet these needs. Assuring access to appropriate, affordable, high-quality services that arewell implemented, however, remains an elusive goal, particularly for many of the mostwww.developingchild.NETdisadvantaged
shaping brain architecture and actually changes the chemistry that encodes the genes in brain cells.30 Put simply, the brain adapts to the expe-riences it has. Certain types of adaptations re-sult in healthy systems, such as effective learning and memory, and other adaptations l