Transcription
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page i Modern ChemistryStudy Guide
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page iiCopyright by Holt, Rinehart and WinstonAll rights reserved. No part of this publication may be reproduced or transmitted inany form or by any means, electronic or mechanical, including photocopy, recording,or any information storage and retrieval system, without permission in writing fromthe publisher.Teachers using MODERN CHEMISTRY may photocopy blackline masters in completepages in sufficient quantities for classroom use only and not for resale.HOLT, MODERN CHEMISTRY, and the “Owl Design” are trademarks licensed toHolt, Rinehart and Winston, registered in the United States of America and/or otherjurisdictions.Printed in the United States of AmericaISBN 0-03-036777-81 2 3 4 5 6 7 8 917007 06 05 04
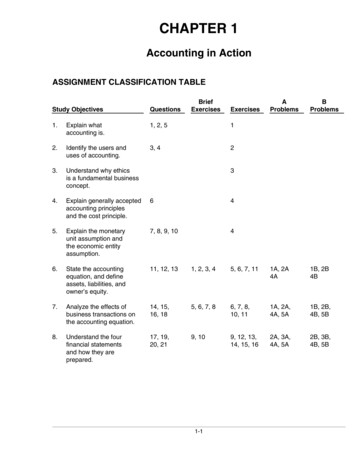
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page iiiContents1 Matter and ChangeSection 1 Chemistry Is a Physical Science. . . . . . . . . . . . . . . . . . . . . 1Section 2 Matter and Its Properties . . . . . . . . . . . . . . . . . . . . . . . . . . 3Section 3 Elements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5Chapter 1 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72 Measurements and CalculationsSection 1 Scientific Method . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9Section 2 Units of Measurement . . . . . . . . . . . . . . . . . . . . . . . . . . . 11Section 3 Using Scientific Measurements . . . . . . . . . . . . . . . . . . . . 12Chapter 2 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153 Atoms: The Building Blocks of MatterSection 1 The Atom: From Philosophical Ideato Scientific Theory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17Section 2 The Structure of the Atom. . . . . . . . . . . . . . . . . . . . . . . . 19Section 3 Counting Atoms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21Chapter 3 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 234 Arrangement of Electrons in AtomsSection 1 The Development of a New Atomic Model . . . . . . . . . . . 25Section 2 The Quantum Model of the Atom . . . . . . . . . . . . . . . . . . 27Section 3 Electron Configurations . . . . . . . . . . . . . . . . . . . . . . . . . . 29Chapter 4 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 315 The Periodic LawSection 1 History of the Periodic Table . . . . . . . . . . . . . . . . . . . . . . 33Section 2 Electron Configuration and the Periodic Table . . . . . . . 35Section 3 Electron Configuration and Periodic Properties . . . . . . 37Chapter 5 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 396 Chemical BondingSection 1 Introduction to Chemical Bonding . . . . . . . . . . . . . . . . . 41Section 2 Covalent Bonding and Molecular Compounds . . . . . . . . 43Section 3 Ionic Bonding and Ionic Compounds . . . . . . . . . . . . . . . 45Section 4 Metallic Bonding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47Section 5 Molecular Geometry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49Chapter 6 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 517 Chemical Formulas and Chemical CompoundsSection 1 Chemical Names and Formulas . . . . . . . . . . . . . . . . . . . . 53Section 2 Oxidation Numbers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55Section 3 Using Chemical Formulas . . . . . . . . . . . . . . . . . . . . . . . . 57Section 4 Determining Chemical Formulas . . . . . . . . . . . . . . . . . . . 59Chapter 7 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61Copyright by Holt, Rinehart and Winston. All rights reserved.Modern ChemistryiiiStudy Guide
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page iv8 Chemical Equations and ReactionsSection 1 Describing Chemical Reactions . . . . . . . . . . . . . . . . . . . . 65Section 2 Types of Chemical Reactions . . . . . . . . . . . . . . . . . . . . . . 67Section 3 Activity Series of the Elements . . . . . . . . . . . . . . . . . . . . 69Chapter 8 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 719 StoichiometrySection 1 Introduction to Stoichiometry . . . . . . . . . . . . . . . . . . . . . 73Section 2 Ideal Stoichiometric Calculations . . . . . . . . . . . . . . . . . . 75Section 3 Limiting Reactants and Percent Yield . . . . . . . . . . . . . . . 77Chapter 9 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7910 States of MatterSection 1 Kinetic Theory of Matter . . . . . . . . . . . . . . . . . . . . . . . . . 81Section 2 Liquids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83Section 3 Solids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85Section 4 Changes of State . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87Section 5 Water . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 89Chapter 10 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9111 GasesSection 1 Gases and Pressure. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 93Section 2 The Gas Laws . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95Section 3 Gas Volumes and the Ideal Gas Law . . . . . . . . . . . . . . . . 97Section 4 Diffusion and Effusion . . . . . . . . . . . . . . . . . . . . . . . . . . . 99Chapter 11 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10012 SolutionsSection 1 Types of Mixtures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103Section 2 The Solution Process . . . . . . . . . . . . . . . . . . . . . . . . . . . 105Section 3 Concentration of Solutions . . . . . . . . . . . . . . . . . . . . . . 107Chapter 12 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10913 Ions in Aqueous Solutions and Colligative PropertiesSection 1 Compounds in Aqueous Solutions. . . . . . . . . . . . . . . . . 111Section 2 Colligative Properties of Solutions . . . . . . . . . . . . . . . . 113Chapter 13 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11514 Acids and BasesSection 1 Properties of Acids and Bases . . . . . . . . . . . . . . . . . . . . 117Section 2 Acid-Base Theories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119Section 3 Acid-Base Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 121Chapter 14 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12315 Acid-Base Titration and pHSection 1 Aqueous Solutions and the Concept of pH . . . . . . . . . . 125Section 2 Determining pH and Titrations . . . . . . . . . . . . . . . . . . . 127Chapter 15 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 129Copyright by Holt, Rinehart and Winston. All rights reserved.Modern ChemistryivStudy Guide
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page v16 Reaction EnergySection 1 Thermochemistry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131Section 2 Driving Force of Reactions . . . . . . . . . . . . . . . . . . . . . . 133Chapter 16 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13517 Reaction KineticsSection 1 The Reaction Process. . . . . . . . . . . . . . . . . . . . . . . . . . . 137Section 2 Reaction Rates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 139Chapter 17 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14118 Chemical EquilibriumSection 1 The Nature of Chemical Equilibrium. . . . . . . . . . . . . . . 143Section 2 Shifting Equilibrium . . . . . . . . . . . . . . . . . . . . . . . . . . . . 145Section 3 Equilibria of Acids, Bases, and Salts . . . . . . . . . . . . . . . 147Section 4 Solubility Equilibrium. . . . . . . . . . . . . . . . . . . . . . . . . . . 149Chapter 18 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15119 Oxidation-Reduction ReactionsSection 1 Oxidation and Reduction . . . . . . . . . . . . . . . . . . . . . . . . 153Section 2 Balancing Redox Equations . . . . . . . . . . . . . . . . . . . . . . 155Section 3 Oxidizing and Reducing Agents . . . . . . . . . . . . . . . . . . . 157Chapter 19 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15920 ElectrochemistrySection 1 Introduction to Electrochemistry . . . . . . . . . . . . . . . . . 161Section 2 Voltaic Cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163Section 3 Electrolytic Cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 165Chapter 20 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16721 Nuclear ChemistrySection 1 The Nucleus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 169Section 2 Radioactive Decay . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 171Section 3 Nuclear Radiation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 173Section 4 Nuclear Fission and Nuclear Fusion . . . . . . . . . . . . . . . 175Chapter 21 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17722 Organic ChemistrySection 1 Organic Compounds . . . . . . . . . . . . . . . . . . . . . . . . . . . . 179Section 2 Hydrocarbons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 181Section 3 Functional Groups . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 183Section 4 Organic Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 185Chapter 22 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18723 Biological ChemistrySection 1 Carbohydrates and Lipids . . . . . . . . . . . . . . . . . . . . . . . 189Section 2 Amino Acids and Proteins . . . . . . . . . . . . . . . . . . . . . . . 191Section 3 Metabolism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 193Section 4 Nucleic Acids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 195Chapter 23 Mixed Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 197Copyright by Holt, Rinehart and Winston. All rights reserved.Modern ChemistryvStudy Guide
mc06se cFMsr i-vi.qxd 11/22/04 12:07 PM Page vi
mc06sete c01sr 001-008.qxd 11/16/04 3:24 PM Page 1(Black plate)NameDateClassCHAPTER 1 REVIEWMatter and ChangeSECTION 1SHORT ANSWER1.Technological development of a chemical product often(a)(b)(c)(d)2.develop new products.make money.understand an environmental problem.gain knowledge.Applied research is designed to(a)(b)(c)(d)4.lags behind basic research on the same substance.does not involve chance discoveries.is driven by curiosity.is done for the sake of learning something new.The primary motivation behind basic research is to(a)(b)(c)(d)3.Answer the following questions in the space provided.solve a particular problem.satisfy curiosity.gain knowledge.learn for the sake of learning.Chemistry is usually classified as(a)(b)(c)(d)a biological science.a physical science.a social science.a computer science.5. Define the six major branches of chemistry.MODERN CHEMISTRYCopyright by Holt, Rinehart and Winston. All rights reserved.MATTER AND CHANGE1
mc06sete c01sr 001-008.qxd 11/16/04 3:24 PM Page 2Name(Black plate)DateClassSECTION 1 continued6. For each of the following types of chemical investigations, determine whether the investigation isbasic research, applied research, or technological development. More than one choice may apply.a. A laboratory in a major university surveys all thereactions involving bromine.b. A pharmaceutical company explores a disease inorder to produce a better medicine.c. A scientist investigates the cause of the ozone hole tofind a way to stop the loss of the ozone layer.d. A pharmaceutical company discovers a moreefficient method of producing a drug.e. A chemical company develops a new biodegradableplastic.f. A laboratory explores the use of ozone to inactivatebacteria in a drinking-water system.7. Give examples of two different instruments routinely used in chemistry.8. What are microstructures?9. What is a chemical?10. What is chemistry?2MATTER AND CHANGEMODERN CHEMISTRYCopyright by Holt, Rinehart and Winston. All rights reserved.
mc06sete c01sr 001-008.qxd 11/16/04 3:24 PM Page 3(Black plate)NameDateClassCHAPTER 1 REVIEWMatter and ChangeSECTION 2SHORT ANSWERAnswer the following questions in the space provided.1. Classify each of the following as a homogeneous or heterogeneous substance.a. iron oreb. quartzc. granited. energy drinke. oil-and-vinegar salad dressingf. saltg. rainwaterh. nitrogen2. Classify each of the following as a physical or chemical change.a. ice meltingb. paper burningc. metal rustingd. gas pressure increasinge. liquid evaporatingf. food digesting3. Compare a physical change with a chemical change.MODERN CHEMISTRYCopyright by Holt, Rinehart and Winston. All rights reserved.MATTER AND CHANGE3
mc06sete c01sr 001-008.qxd 11/16/04 3:24 PM Page 4(Black plate)NameDateClassSECTION 2 continued4. Compare and contrast each of the following terms:a. mass and matterb. atom and compoundc. physical property and chemical propertyd. homogeneous mixture and heterogeneous mixture5. Using circles to represent particles, draw a diagram that compares the arrangement of particles inthe solid, liquid, and gas states.SolidLiquidGas6. How is energy involved in chemical and physical changes?4MATTER AND CHANGEMODERN CHEMISTRYCopyright by Holt, Rinehart and Winston. All rights reserved.
mc06sete c01sr 001-008.qxd 11/16/04 3:24 PM Page 5(Black plate)NameDateClassCHAPTER 1 REVIEWMatter and ChangeSECTION 3SHORT ANSWERAnswer the following questions in the space provided.1. A horizontal row of elements in the periodic table is called a(n).2. The symbol for th
choices: organic chemistry, analytical chemistry, biochemistry, theoretical chemistry. More than one branch may be appropriate. a. A forensic scientist uses chemistry to find information at the scene of a crime. b. A scientist uses a computer model to see how an enzyme will function. c. A professor explores the reactions that take place in a human liver. d. An oil company scientist tries to .