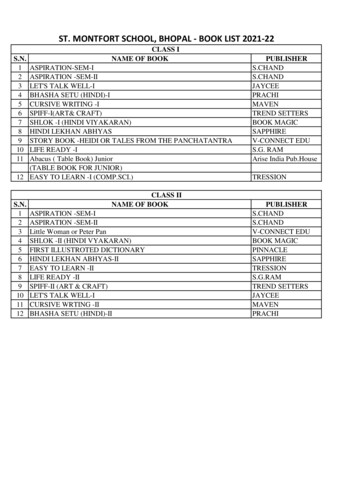
Transcription
NCERT Physics (Vol 2)Chapter 3 Thermal properties of matterCBSE NCERT Solutions for Class 11 physics Chapter 3ExercisesQ.1.The following questions based on the P-T phase diagram of carbon dioxide:At what temperature and pressure can the solid, liquid and vapour phases of CO2 co-exist in equilibrium?Solution:Q.2.The following questions based on the P-T phase diagram of carbon dioxide:What is the effect of the decrease of pressure on the fusion and boiling point of CO2Solution:Q.3.It can be seen that the three curves intersect at point C. Therefore, point C is the triple point of the CO2. This means that at thepressure and temperature corresponding to this point (i.e., at -56.6 oC and 5.11 atm), the solid phase,liquid phase and vapour phases of CO2 co-exist in equilibrium.Fusion point is the temperature at which liquid turns into solid, and boiling point is the temperature at which liquid turns intogas. It can be observed from the graph that, both these temperatures decrease as the pressure is decreased.The following questions based on the P-T phase diagram of carbon dioxide:What are the critical temperature and pressure for CO2? What is their significance?Solution:Critical point is the point at which the liquid-vapour curve terminates. Therefore, critical temperature and critical pressureof CO2 are 31.1 oC and 73 atm respectively. Beyond this temperature, even if a lot of pressure is applied, CO2 cannot beconverted into liquid form.Practice more on Thermal properties of matterPage 1www.embibe.com
NCERT Physics (Vol 2)Q.4.Chapter 3 Thermal properties of matterThe following questions based on the P-T phase diagram of carbon dioxide:Is CO2 solid, liquid or gas at (a) -70 oC under 1 atm, (b) -60 oC under 10 atm, (c) 15 oC under 56 atm?Solution:It can be concluded from the P-T phase diagram of CO2that:(a) CO2 at -70oC, under 1 atm pressure exists only in gaseous state(b) CO2 at -60oC, under 10 atm pressure can exist as solid or vapour depending on the value of pressure. (c) CO2 at 15oC,under 56 atm pressure can exist as liquid or vapour depending on the value of pressure.Q.5.Answer the following questions based on the P–T phase diagram of CO2:CO2 at 1 atm pressure and temperature -60 oC is compressed isothermally. Does it go through a liquid phase?Solution:From the given phase diagram:We can conclude that it doesn't go through liquid phase. At 1 atm pressure and at -60 oC,CO2 exists in vapour state, and it liesto the left of -56.6 oC (triple point C). when the gas is compressed, i.e., more pressure is applied, it can be seen form the graphthat it enters the solid phase directly.Practice more on Thermal properties of matterPage 2www.embibe.com
NCERT Physics (Vol 2)Q.6.Chapter 3 Thermal properties of matterAnswer the following questions based on the P–T phase diagram of CO2What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure?Solution:Q.7.It condenses to solid directly.At 4 atm lies below triple point (5.11 atm). Therefore, it exists either in the vapour or solid phase. It therefore condensesstraight into the solid state, without going through the liquid state.Answer the following questions based on the P–T phase diagram of CO2Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and –65 oC temperature as it is heated up to roomtemperature at constant pressure.Solution:Q.8.Draw a line parallel to Y axis passing through 10 atm. As the temperature is increased from -65 C, the points on the line areobserved to go from solid region to liquid region to vapour region. The fusion and boiling points are given by the intersectionpoints where this parallel line cuts the fusion and vaporisation curves respectively.Answer the following questions based on the P–T phase diagram of CO2CO2 is heated to a temperature 70 oC and compressed isothermally. What changes in its properties do you expect to observe?Solution:Q.9.If CO2 is heated to 70 oC and compressed isothermally, then it will not exhibit any transition to the liquid state. This isbecause 70 oC is higher than the critical temperature of CO2 . Therefore, it will remain in the gaseous state but as pressureincreases, its behaviour departs more and more from ideal gas behaviour.The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius andFahrenheit scales.Practice more on Thermal properties of matterPage 3www.embibe.com
NCERT Physics (Vol 2)Solution:Chapter 3 Thermal properties of matterThe Kelvin and Celsius's scales are related as:TK TC 273.15 . (i)Celsius and Fahrenheit's scales are related as:TF 95TC 32 . (ii)For neon:TK 24.57 K Tc 24.57–273.15 –248.58 CTF 95Tc 32 95-248.58 32 -415.44 oFFor carbon dioxide:TK 216.55 K TC 216.55-273.15 -56.60 oCTF 95Tc 32 95-56.60 32 -69.88 oFQ.10.A brass rod of length 50 cm and diameter 3 mm is joined to a steel rod of the same length and diameter. What is the change in length of thecombined rod at 250 C, if the original lengths are at 40.0 C? Is there a ‘thermal stress’ developed at the junction? The ends of the rod arefree to expand (Co-efficient of linear expansion of brass is 2.0 10-5 K-1 and steel is 1.2 10-5 K-1)Solution:Q.11.Initial temperature, T1 40 CFinal temperature, T2 250 CChange in temperature, ΔT T2-T1 210 CLength of the brass rod, l1 50 cmLength of the steel rod at l2 50 cmCoefficient of linear expansion of brass, α1 2.0 10-5 K-1Coefficient of linear expansion of steel, α2 1.2 10-5 K-1Total change in length is the summation of change in lengths of each of rods.Therefore, l l1 l2 (l1α1 l2α2)(T2-T1) (50 2.0 10-5 50 1.2 10-5)(250-40)There will not be any thermal stress as the rods are free to expand. 50 3.2 10-5 210 0.336 cmThe coefficient of volume expansion of glycerin is 49 10-5 K-1. What is the fractional change in its density for a 30 C rise in temperature?Solution:Given,Coefficient of volume expansion, γ 49 10-5 K-1Rise in temperature, t 30 CWe know that,ρ2 ρ1(1 γ t) ρ1(1 -γ t) ρ2-ρ1ρ1 -γ tTherefore, fractional change in density 49 10-5 30 1.47 10-2Q.12.A 10 kW drilling machine is used to drill a bore in a small aluminium block of mass 8.0 kg. How much is the rise in temperature of theblock in 2.5 minutes, assuming 50 % of power is used up in heating the machine itself or lost to the surroundings? Specific heat ofaluminium 0.91 Jg-1K-1.Wherever ApplicableSolution:Power of the drilling machine, P 10 kW 104 WMass of the aluminium block, m 8.0 kg 8000 gTime for which the machine is used, t 2.5 min 150 sSpecific heat of aluminium, c 0.91 Jg-1K-1Say, the Rise in the temperature of the block after drilling is δTThe total energy spent by the drilling machine in the given time is,E Pt 104 150 1.5 106 JIt is given that 50% of this is converted into heat.Hence, heat, Q 50100 1.5 106 7.5 105 JBut Q mc T T Qmc 7.5 1058000 0.91 103.02 CHence, in 2.5 minutes of drilling, the rise in the temperature of the block is 103.02 oC.Q.13.A copper block of mass 2.5 kg is heated in a furnace to a temperature of 500 C and then placed on a large ice block. What is the maximumamount of ice that can melt? (Specific heat of copper 0.39 Jg-1K-1; the heat of fusion of water is 335 Jg-1).Practice more on Thermal properties of matterPage 4www.embibe.com
NCERT Physics (Vol 2)Solution:Chapter 3 Thermal properties of matterSince there is large amount of ice, the equilibrium temperature will be 0 C.Mass of the copper block, m 2.5 kg 2500 gThe Rise in the temperature of the copper block, Δθ 500 CSpecific heat of copper, C 0.39 Jg-1 C-1The heat of fusion of water, L 335 Jg-1Maximum heat the copper block can lose, Q mCΔθ 2500 0.39 500 487500 JLet m1 be the amount of ice that melts when the copper block is placed on it.Hence, the heat gained by the melted ice, Q m1L 487500 m1 335 m1 487500335 1.45 kgQ.14.In an experiment on the specific heat of a metal, a 0.20 kg block of the metal at 150 C is dropped in a copper calorimeter (of waterequivalent 0.025 kg) containing 150 cm3 of water at 27 C. The final temperature is 40 C. Compute the specific heat of the metal. If heatlosses to the surroundings are not negligible, is your Solution: greater or smaller than the actual value for the specific heat of the metal?Solution:Mass of the metal, m 0.20 kg 200 gThe initial temperature of the metal, T1 150 CThe calorimeter has water equivalent of mass, m' 0.025 kg 25 gThe volume of water, V 150 cm3So, mass of water, M 150 gThe combined water equivalent of calorimeter and water is 25 150 175 g 0.175 kgSpecific heat of water, C 4186 Jkg-1K-1The initial temperature of the water, T2 27 CThe final temperature of the mixture, T3 40 CHeat gained by calorimeter and water,Q mC T 0.175 4186 (40-27) 9523.15 JHeat lost by metal,Qm 0.2 C (150-40) 22CHeat lost is equal to heat gained. 9523.15 22C C 9523.1522 432.87 Jkg-1K-1Q.15.Given below are observations on molar specific heats at room temperature of some common gases.GasMolar specific heat (Cv) cal mol-1K-1Hydrogen4.87Nitrogen4.97Oxygen5.02Nitric oxide4.99Carbon monoxide 5.01Chlorine6.17The measured molar specific heats of these gases are markedly different from those for monatomic gases. Typically, molar specific heat of amonatomic gas is 2.92cal/mol K. Explain this difference. What can you infer from the somewhat larger (than the rest) value for chlorine?Solution:Q.16.For a monoatomic ideal gas, there are three degrees of freedom, so the specific heat is given by, Cv 32RFor a diatomic gas, there are five degrees of freedom, so the specific heat is given by, Cv 52RIt can be seen that the specific heat for diatomic gas is 53 times that of monoatomic gas.All the given gases are diatomic, and therefore this difference can be seen.Chlorine has more specific heat than other diatomic molecules because it has additional degrees of freedom in the form ofvibration.A child running a temperature of 101 oF is given an antipyrine (i.e. medicine that lowers fever) which causes an increase in the rate ofevaporation of sweat from his body. If the fever is brought down to 98 oF in 20 min, what is the average rate of extra evaporation caused,by the drug? Assume the evaporation mechanism to be the only way by which heat is lost. The mass of the child is 30 kg. The specific heatof the human body is approximately the same as that of water, and latent heat of evaporation of water at that temperature is about580 calg-1Practice more on Thermal properties of matterPage 5www.embibe.com
NCERT Physics (Vol 2)Solution:Chapter 3 Thermal properties of matterThe initial temperature of the body of the child, T1 101 oFThe final temperature of the body of the child, T2 98 FChange in temperature, ΔT 101-98 59 C 53 CTime taken to reduce the temperature, t 20 minMass of the child, m 30 kg 30000 gThe Specific heat of the human body, C 1 cal g-1 C-1Latent heat of evaporation of water, L 580 cal g-1The heat lost by the child is given as: Q mC T 30000 1 53 50000 calLet m1 be the mass of the water evaporated from the child’s body in 20 min.Loss of heat through water is given by: Q m1L m1 QL 50000580 86.2 g The average rate of extra evaporation caused by the drug m1t 86.220 4.31 g min-1Q.17.A ‘thermocol’ ice box is a cheap and efficient method for storing small quantities of cooked food in summer in particular. A cubical iceboxof side 30 cm has a thickness of 5.0 cm. If 4.0 kg of ice is put in the box, estimate the amount of ice remaining after 6 h. The outsidetemperature is 45 oC, and co-efficient of thermal conductivity of thermocol is 0.01 Js-1m-1K-1. [Heat of fusion of water 335 103 Jkg-1]Solution:Side of the given cubical ice box, s 30 cm 0.3 mThe thickness of the icebox, l 5.0 cm 0.05 mSurface area of the box, A 6s2 6 0.32 0.54 m2Mass of ice kept in the icebox, m 4 kgTime gap, t 6 h 21600 sOutside temperature, T 45 oCCoefficient of thermal conductivity of thermocol, K 0.01 Js-1m-1K-1The heat of fusion of water, L 335 103 Jkg-1Let mi be the total amount of ice that melts in 6 h. The amount of heat lost by the food is given by:Q KA Tl t 0.01 0.54 455 10-2 21600 104976 JHeat gained by ice,Qi miL mi QiL 104976335 103 0.313 kgMass of ice left 4 - 0.313 3.687 kgQ.18.A brass boiler has a base area of 0.15 m2 and thickness of 1.0 cm. It boils water at the rate of 6.0 kg/min when placed on a gas stove.Estimate the temperature of the part of the flame in contact with the boiler. Thermal conductivity of brass is 109 Js-1m-1K-1; Heat ofvaporisation of water is 2256 103 Jkg-1.Solution:The base area of the boiler, A 0.15 m2The thickness of the boiler, l 1.0 cm 0.01 mBoiling rate of water, R 6.0 kg/min 660kg/s 0.1 kg/sThermal conductivity of brass, K 109 Js-1m-1K-1The heat of vaporisation, L 2256 103 Jkg-1Say, the temperature of the flame is TRate of heat taken by water,Hw R L 0.1 2256 103 225600 J/sThe amount of heat flowing into the water through the brass base of the boiler is given by:Hb KAT1-T2l 109 0.15 (T - 100)0.01 1635 (T - 100) Js-1Heat taken by water is equal to heat given by brass boiler. Hb Hw 1635 (T - 100) 225600 T - 100 137.98 T 237.98 CQ.19.Explain why a body with large reflectivity is a poor emitter.Solution:Q.20.Explain why a brass tumbler feels much colder than a wooden tray on a chilly day.Solution:Q.21.A body which has large reflectivity absorbs lesser. A body which absorbs lesser should also emit lesser; otherwise, it keepsloosing heat forever and can never reach equilibrium. Therefore, a body which has good reflectivity should be a poor emitter.Brass is an excellent heat conductor. When a brass tumbler is touched, heat is easily driven from the body to the brass tumbler.As a result, the body's temperature decreases to a lesser value and one feels cooler. Wood is a bad heat conductor. When awooden tray is touched, very little heat is carried from the body to the wooden tray. Therefore, the temperature drop of thebody is only negligible and one does not feel cool. Thus, a brass tumbler feels colder than a wooden tray on a chilly day.Explain why: An optical pyrometer (for measuring high temperatures) calibrated for ideal black body radiation gives too low a value for thetemperature of a red-hot iron piece in the open, but gives a correct value for the temperature when the same piece is in the furnace.Solution:When an optical pyrometer is put in open, it loses heat quickly to the surroundings, as heat radiated is given by,E σT4-T04, where T0 is the surrounding temperature. As T0 is lesser in case of open environment, the rod loses heat quickerand the temperature goes down faster.Practice more on Thermal properties of matterPage 6www.embibe.com
NCERT Physics (Vol 2)Q.22.Explain why the earth without its atmosphere would be inhospitably coldSolution:Q.23.Chapter 3 Thermal properties of matterThe earth would be inhospitably cold without its atmosphere. The atmosphere reflects the radiation emitted by the earth back toits surface keeping it warm.Explain why heating systems based on circulation of steam are more efficient in warming a building than those based on circulation of hotwater.Solution:A steam-based heating system is more efficient in heating a building than a system based on hot water circulation. This isbecause steam in the form of latent heat (540 cal/g) contains surplus heat.Q.24.Two absolute scales A and B have triple points of water defined to be 200 A and 350 B. What is the relation between TA and TB?Solution:Both are absolute scales, so their zero values are same. Therefore,200 - TATA-0 350 - TBTB-0 TA TB47Q.25.A body cools from 80 oC to 50 oC in 5 minutes. Calculate the time it takes to cool from 60 oC to 30 oC. The temperature of thesurroundings is 20 oC.Solution:According to Newton’s law of cooling:dTdt -KT-T0dTT-T0 -Kdtwhere T0 is the sorrounding temperature.Up on integrating and applying limits, we get, T1T2dTT-T0 -Kt lnT2-T0T1-T0 -KtGiven that the body cools from 80 C to 50 C in 5 mins when the sorroundings are at 20 C, ln50 - 2080 - 20 -5K K 15ln2The body cools from 60 C to 30 C in,ln30 - 2060 - 20 -15ln2t t 10 minsQ.26.The electrical resistance in ohms of a certain thermometer varies with temperature according to the approximate law:R Ro1 αT-ToThe resistance is 101.6 Ω at the triple-point of water 273.16 K, and 165.5 Ω at the normal melting point of lead 600.5 K. What is thetemperature when the resistance is 123.4 Ω?Solution:It is given that:R R01 αT-T0 (i)Where,R0 and T0 are the initial resistance and temperature respectively. R, and T are the final resistance and temperaturerespectively. α is a constant.At the triple point of water, T0 273.15 KThe resistance, R0 101.6 ΩAt normal melting point of lead, T 600.5 KThe resistance, R 165.5 ΩPutting these values in equation (i), we get:R R01 αT-T0165.5 101.61 α600.5-273.151.629 1 α327.35 α 0.629327.35 1.92 10-3 K-1For resistance, R1 123.4 ΩR1 R01 αT-T0Where T is the temperature when the resistance is 123.4 Ω123.4 101.61 1.92 10-3T-273.151.214 1 1.92 10-3T-273.150.2141.92 10-3 T-273.15 T 384.61 KQ.27.The triple-point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and theboiling point of water as standard fixed points (as was originally done in the Celsius scale) ?Solution:Q.28.The triple point of water is always at 273.16 k and only at a particular pressure. So value of the triple point is always the sameand it is independent of the pressure. However, the boiling and melting points are dependent on pressure, so they are lessreliable than triple point.There were two fixed points in the original Celsius scale which were assigned the number 0 C and 100 C respectively. On the absolutescale, one of the fixed points is the triple-point of water, which on the Kelvin absolute scale is assigned the number 273.16 K. What is theother fixed point on the (Kelvin) scale ?Solution:The absolute zero temperature is the other fixed point for the Kelvin scale. The absolute temperature is assigned a value of 0 K.Practice more on Thermal properties of matterPage 7www.embibe.com
NCERT Physics (Vol 2)Q.29.Chapter 3 Thermal properties of matterThe absolute temperature (Kelvin scale) T is related to the temperature Tc on the Celsius scale byTc T – 273.15Why do we have 273.15 in this relation and not 273.16?Solution:The temperature of 273.16 K is the triple point of water. It is not the melting point of ice. The temperature 0 C on the Celsiusscale is the melting point of ice. Its corresponding value on the Kelvin scale is 273.15 KAccordingly, absolute temperature (Kelvin scale) T is related to Celsius scale as: Tc T-273.15Q.30.What is the temperature of the triple-point of water on an absolute scale whose unit interval size is equal to that of the Fahrenheit scale?Solution:Gap between freezing point and melting point of water in F is 180 and in K is 100.Therefore, 1 F rise is equal to 100180 rise in K.Given that, the unit interval size of the new absolute scale is same as Fahrenheit scale,so 1 unit rise in the absolute scale is equal to 100180units rise in K.Or, 1 unit rise in K is equal to 180100 units rise in the new absolute scale. 237.16 units rise in K is equal to 237.16 180100 491.69 units rise in the absolute scale.Since, both the scales start from zero at the same temperature,273.16 K is equal to 491.69 units.Q.31.Two ideal gas thermometers A and B use oxygen and hydrogen, respectively. The following observations are made:TemperaturePressure thermometer A Pressure thermometer BTriple-point of water1.250 105 Pa0.200 105 PaNormal melting point of sulphur 1.797 105 Pa0.287 105 PaWhat is the absolute temperature of the normal melting point of sulphur as read by thermometers A and B? What do you think is the reasonbehind the slight difference in answers of thermometers A and B ? (The thermometers are not faulty). What further procedure is needed inthe experiment to reduce the discrepancy between the two readings ?Solution:The triple point of water, T 273.16 K.At this temperature, pressure in thermometer A, PA 1.250 105 PaLet T1 be the normal melting point of sulphur.At this temperature, pressure in thermometer A, P1 1.797 105 PaAccording to Charles’ law:PAT P1T1 T1 P1TPA 1.797 105 273.161.250 105 392.69 KThe absolute temperature of the normal sulphur melting point as read by thermometer A is therefore 392.69 KAt triple point 273.16 K, the pressure in thermometer B, PB 0.200 105 PaAt temperature T1, the pressure in thermometer B, P2 0.287 105 PaAccording to Charles’ law:PBT P1T10.200 105273.16 0.287 105T1 T1 0.287 1050.200 105 273.16 391.98 KThe absolute temperature of the normal sulphur melting point as read by thermometer A is therefore 391.98 KThe difference in answers in both the cases is because the gases are not ideal. They are real gases, and they deviate from idealgas behaviour. To increase the accuracy of the readings, the initial pressure of the gases can be reduced to a very low value byremoving some gas from the thermometers. Real gases behave more like ideal gases at very low pressures.Q.32.Two ideal gas thermometers A and B use oxygen and hydrogen respectively. The following observations are made :TemperatureTriple-point of waterNormal melting point ofsulphurPressure thermometer Pressure thermometerAB1.250 105 Pa0.200 105 Pa1.797 105 Pa0.287 105 Pa(b) What do you think is the reason behind the slight difference in answers of thermometers A and B ? (The thermometers are not faulty).What further procedure is needed in the experiment to reduce the discrepancy between the two readings ?Solution:Q.33.The oxygen and hydrogen gas contained in the A and B thermometers are not ideal gases. Therefore, the measurements ofthermometers A and B differ slightly. The experiment should be carried out under low-pressure circumstances to decrease thediscrepancy between the two measurements. These gases act as perfect ideal gases at low pressure.A steel tape 1 m long is correctly calibrated for a temperature of 27.0 oC. The length of a steel rod measured by this tape is found to be63.0 cm on a hot day when the temperature is 45.0 oC. What is the actual length of the steel rod on that day? What is the length of the samePractice more on Thermal properties of matterPage 8www.embibe.com
NCERT Physics (Vol 2)Chapter 3 Thermal properties of mattersteel rod on a day when the temperature is 27.0 oC? Coefficient of linear expansion of steel 1.20 10-5 K-1.Solution:Both the rod and the tape are made of steel. So, even if the temperature rises, the measurement of the rod by the tape remainsthe same as both of them expand in similar proportions. Given that the tape measured the rod as 63 cm at 45 C, it measures thesame at 27 C also. Since the tape is calibrated at 27 C, the measurement made by the tape at that temperature is the correctmeasurement. Therefore the correct length of the rod at 27 C is 63 cm.Length of the steel rod at 27 C is 63 cmLength of the rod at 45 C will be,l l0(1 α t) 63 (1 1.2 10-5 (45 - 27)) 63.0136 cmQ.34.A large steel wheel is to be fitted onto a shaft of the same material. At 27 C, the outer diameter of the shaft is 8.70 cm, and the diameter ofthe central hole in the wheel is 8.69 cm. The shaft is cooled using ‘dry ice’. At what temperature of the shaft does the wheel slip on theshaft? Assume the coefficient of linear expansion of the steel to be constant over the required temperature range: αsteel 1.20 10-5 K-1Solution:Q.35.A hole is drilled in a copper sheet. The diameter of the hole is 4.24 cm at 27.0 C. What is the change in the diameter of the hole when thesheet is heated to 227 C? Coefficient of linear expansion of copper 1.70 10-5 K-1Solution:Q.36.The given temperature, T 27 C 300 KThe outer diameter of the steel shaft at T, d1 8.70 cmCoefficient of linear expansion of steel, αsteel 1.20 10-5K-1After the shaft is cooled using ‘dry ice’, say, its temperature becomes T1The desired diameter of the shaft at T1 is, d2 8.69 cm.Therefore,d2 d1 (1 α(T1 - T) ) 8.69 8.7 (1 1.2 10-5 (T1-300)) T1-300 -95.78 K T1 204.21 K T1 -68.78 CInitial temperature, T1 27.0 CThe diameter of the hole at T1, d1 4.24 cmFinal temperature, T2 227 CSay, the diameter of the hole at T2 is d2.Co-efficient of linear expansion of copper, αCu 1.70 10-5 K-1Therefore,d2 d1(1 αCu(T2-T1)) 4.24 (1 1.7 10-5 (227 - 27)) 4.24 (1 0.0034) 4.2544 cmA brass wire 1.8 m long at 27 C is held taut with little tension between two rigid supports. If the wire is cooled to a temperature of -39 C,what is the tension developed in the wire, if its diameter is 2.0 mm? Co-efficient of linear expansion of brass is 2.0 10-5 K-1; Young’smodulus of brass is 0.91 10 Pa.Solution:Length of the brass wire at T1, l 1.8 mFinal temperature, T2 -39 CThe diameter of the wire, d 2.0 mm 2 10-3 mSay, tension developed in the wire is FThe Coefficient of linear expansion of brass, α 2.0 10-5 K-1Young’s modulus of brass, Y 0.91 1011 PaThe change in natural length due to change in temperature is, l lα(T2-T1) l 2 10-5 (-39-27) l 132 10-5Therefore, the rigid supports are expanding the wire by this amount.The area of cross section of the wire, A πd2/4 3.14 10-6 m2By applying the relation between stress and strain using Young's modulus, we get,F YA ll 0.91 1011 3.14 10-6 l 132 10-5l 377.176 NThis is a tensile force since the rod is under expansion.Practice more on Thermal properties of matterPage 9www.embibe.com
NCERT Physics (Vol 2)Practice more on Thermal properties of matterChapter 3 Thermal properties of matterPage 10www.embibe.com
The combined water equivalent of calorimeter and water is 25 150 175 g 0.175 kg Specific heat of water, C 4186 Jkg-1K-1 The initial temperature of the water, T2 27 C The final temperature of the mixture, T3 40 C Heat gained by calorimeter and water, Q mC T .175 4186 (40-27) 9523.15 J Heat lost by metal,