Transcription
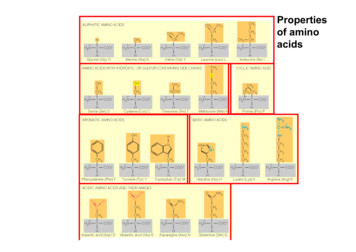
Propertiesof aminoacids
Primary structure:Sequence of amino acids within the peptide chain.Peptide bondsAmino-terminal endCarboxy-terminal end
The α-helix II¾ The peptide bondcauses a dipole whichbecomes stronger by theregular arrangement¾ Consequence:Positively charged Nterminal endNegatively charged Cterminal endAtome: rot, Sauerstoff;schwarz: Kohlenstoff;blau: Stickstoff; weiß;Wasserstoff.Biochemie/Proteine 283
Carbohydrates¾ The simplest compound with the gross fomula (CH2O)n is formaldehyde: n 1¾ H2C O has little in common with usual concept of sugars¾ Smallest carbohydrates are the trioses¾There are two trioses: Glyceraldehyde and dihydroxyacetone¾ Represent major classes of monosaccharides¾ Glyceraldehyde is an aldehyde: Aldoses¾ Dihydroxyacetone is a keton: Ketoses¾ Tautomer structures¾ Can interconvert through an instable intermediate: EnediolMathews, van Holde & Ahern, 2000
Ring structuresExample: ribose, a pentose¾ Can form either a five-membered furanose ring¾ Or a six-membered pyranose ring¾ Reaction: formation of hemiacetals from the acldehyde group¾ In each case, two enantiomeric forms, α or ß are possibleNucleophilicattackMathews, van Holde & Ahern, 2000
Hexose ringsPyranose ring conformations¾ Chair (more stable)¾ Boat (less stable)Mathews, van Holde & Ahern, 2000
Same monomers, different polymers:h Starch (α-1,4 bonds)h Cellulose (ß-1,4 bonds)h Glycogen (α-1,4 and α-1,6 bonds)
Starch (Stärke)Celluloseß-1,4 -1,4CellobioseInfluence of binding form on secondary structureCellulose is the most abundantbiopolymer on earth
GlycogenBranched polysaccharide: The linkages betweenglucose residues are of two types, α-1,4 and α-1,6Glycogen inliver cellsStorage polymercomposed ofglucose inanimal liver
Murein: Poly-N-acetyl glucosamine – theconstruction material for bacterial cell walls
Smooth and rough colonies of B. anthracis
Sugars and blood groupsThe ABO blood group antigensThe O oligosaccharide does not elicitantibodies in most humansThe A and B antigens are formed byaddition of GalNAc or Gal, respectively,to the O oligosaccharideEach of the A and B antigens can elicit aspecific antibodyIn this figure, R can represent either aprotein molecule or a lipid moleculeMathews, van Holde & Ahern, 2000
Phospholipids
Hydrophobic Bonds („hydrophobic interactions“)¾ Non-polar molecules mixed withwater do not dissolve (e.g. oil slick onwater). Why?¾ Water is held together by hydrogenbonds. If nonpolar molecule is insertedinto water, would have to break theordered lattice of water molecules heldtogether by H bonds.¾ But this would require energy – itcanott happen spontaneously. Instead,nonpolar molecules (or parts ofmolecules) will aggregate to avoidwater.¾ A similar situation occurs in parts ofmany proteins.¾ The Hydrophobic Bond is very weak(ca. 2,5 J Mol-1), rather a lower energystate than would occur if thesemolecules were dissolved in water.
Functions of membranes
Fluid mosaic modelDiagram of the structure of the cytoplasmic membrane; the inner surface (In) faces the cytoplasm andthe outer surface (Out) faces the environment.¾ The matrix of the unit membrane is composed of phospholipids, with the hydrophobic groups directed inwardand the hydrophilic groups toward the outside, where they associate with water.¾ Embedded in the matrix are proteins that have considerable hydrophobic character in the region thattraverses the fatty acid bilayer.¾ Hydrophilic proteins and other charged substances, such as metal ions, may be attached to the hydrophilicsurfaces.¾ Although there are some chemical differences, the overall structure of the cytoplasmic membrane shown issimilar in both prokaryotes and eukaryotesBrock, 10th ed.
Pyrimidine basesSugar binding sitesPurine basesSugar binding sitesStructure of the bases of D N A and RNA. The letters, C, T, U, A and G are usedto designate the individual bases. In attaching the base to the 1‘ carbon of thesugar phosphate, pyrimidine bases are bonded through N-1 of the ring andpurine bases through N-9 of the ring
Structural formulas of nucleic acid sugars.The name „deoxy“comes because herean O is lacking¾ The formulas can be represented in two alternate ways, open chain and ring.¾ The open chain is easier to visualize, but the ring form is the commonly usedstructure.¾ Note the numbering system on the ring.
This is a nucleosideThis is a nucleotide
5‘ end3‘ end5‘ end3‘ endPrimary structure of nucleicacids: base sequence
G-C: 3 hydrogen bondsSecondary structure ofnucleic acids: Base pairs2 hydrogen bonds
The tertiary structure of nucleic acidsFranklin's X-ray diagram of the B formof sodium thymonucleate (DNA) fibres,published in Nature on 25 April 1953,shows "in striking manner the featurescharacteristic of helical structures"5.„Her photographs are among the mostbeautiful X-ray photographs of anysubstance every taken." — J. D. Bernal,1958.From the Nobel prize winning publication in Nature
Quarternary structureof DNA: supercoilingRelaxed and supercoiled DNA
Melting and denaturizing of DNA¾ A T pairs are more easilyseparated than G C pairs¾ Therefore, denaturation startswith A T pairs¾ High G C proportion: highermelting point
RNA: ribonucleic acid – single stranded Backbone:Ribose,Phosphoric acid Bases: A,G,C,U single stranded 80-85% ofbacterial RNA is inribosomes Ribosomes:Protein synthesis Informationencoded on DNAis translated intoprotein via RNAduring proteinsynthesis
Three major types of RNA messenger RNA (mRNA) transfer RNA (tRNA) ribosomal RNA (rRNA) Two types of function genetic- carries genetic information of DNA (mRNA) structurale.g. -structural role in ribosome (rRNA),- amino acid transfer (tRNA),- catalytic (enzymatic) activity (ribozymes)PD Dr. Bettina Siebers
The UV damage to DNAUltraviolet (UV) photons harm the DNA molecules of living organisms in differentways. In one common damage event, adjacent bases bond with each other,instead of across the “ladder.”This makes a bulge, and the distorted DNA molecule does not function properly.(Illustration by David n/thy dmr.gif
ATPComponents of the important nucleotide, adenosine triphosphate (ATP)The energy of hydrolysis of a phosphoanhydride bond (shown as squiggles) is greaterthan that of a phosphate ester and will have significance in Chapter 5 (Section 5.8)[Brock, 10th ed.]
NAD /NADHCoenzymeRequired for redox reactionsExample: Reduction of pyruvate
Although not used in the electron transport chain, Coenzyme A is a major cofactor which is usedto transfer a two carbon unit commonly referred to as the acetyl group. The structure has manycommon features with NAD and FAD in that it has the diphosphate, ribose, and adenine. Inaddition it has a vitamin called pantothenic acid, and finally terminated by a thiol group. The thiol(-SH) is the sulfur analog of an alcohol (-OH).The acetyl group (CH3C O) is attached to the sulfur of the CoA through a thiol ester type bond.Acetyl CoA is important in the breakdown of fatty acids and is a starting point in the citric acidcyclewww.elmhurst.edu/ chm/vchembook/images/594CoA.gif
Structural formulas of nucleic acid sugars. . From the Nobel prize winning publication in Nature. Relaxed and supercoiled DNA Quarternary structure of DNA: supercoiling. ¾A T pairs are more easily separated than G C pairs ¾Therefore, denaturation starts with A T pairs ¾High G C proportion: higher melting point Melting and denaturizing of DNA Backbone: Ribose, Phosphoric acid .