Transcription
Welcome toTechnology Transfer TacticsBelow is your FREE sample issue of our flagshipmonthly newsletter.Enjoy this issue filled with detailed, actionable information and advice with a veryspecific and single‐minded goal: To help you find, develop, license, and bring tomarket your organization’s valuable intellectual property.After you have reviewed this issue, become a SUBSCRIBER and save 250off the subscription price – you pay only 447!We’ll also send you Invention Disclosure Management: Proven Strategiesfor Boosting Quantity and Assessing Quality, a 3‐session collection fromour distance learning professional development series. This best‐sellingmarketing bonus is regularly priced at 379, BUT YOU’LL GET IT FREEwhen you become a subscriber.CLICK HERE to Become a Technology TransferTactic Subscriber Today!Or Phone: Toll‐FREE 1‐877‐729‐0959 or (239) 263‐0605
Technology Transfer TacticsTMThe monthly advisor on best practices in technology transferIn This Issueu TTOs link with third parties in bid toexpand licensing of research tools.While it is always a challenge to motivatefaculty to disclose their innovations,many observers agree it is particularlydifficult when it comes to the disclosureof research tools -- mouse models, celllines, antibodies, and so forth . p. 177u Hopkins collaborates with investmentfirm to de-risk early-stage therapeutics. The Johns Hopkins University andinvestment advisor DeerfieldManagement have launched a clever collaboration called Bluefield Innovationsthat will sponsor research into earlystage therapeutics then license theresults . p. 177u Attorney: Don’t put too much faith insovereign immunity. Sovereign immunity can add to the value of patents ownedby state universities, but it is not an ironclad defense against patent challenges -at least not until we hear more from thecourts, an attorney cautions . p. 178u Creative solutions found for improving reporting between IIA participants. In any organization whereresources are limited, choices must becontinually made on the allocation ofthose resources. In TTOs, shortages oftime and staffing are common, and lackof either or both can cause someprocesses to fall by the wayside p. 179u UTRF makes deal with defensivepatent aggregator RPX. A deal betweenthe University of Tennessee ResearchFoundation and a patent aggregatorillustrates one possible outcome when auniversity asserts patents. Rather thansettling an infringement matter throughlitigation, a defensive patent aggregatormay step in and negotiate a deal to purchase the patents and take the case outof the courtroom . p. 189u Tech Navigator program helps guideNew Mexico entrepreneurs. Gettingnew technology to market in New Mexicois getting a helping hand thanks to a newprogram -- Tech Navigator -- which ishelping to connect the region’s best andbrightest in technology commercialization . p. 190TTOs link with third partiesin bid to expand licensingof research toolsWhile it is always a challenge to motivate faculty to disclose their innovations, many observers agree it is particularlydifficult when it comes to the disclosure of research tools -mouse models, cell lines, antibodies, and so forth. “We at UGAhave perceived a lot of lack of responsiveness from ourresearchers to our requests for RT (research tool) disclosures,as opposed to the more expeditious filing of disclosures ofmore complex technologies,” notes Gennaro J. Gama, PhD,senior technology manager at the University of GeorgiaResearch Foundation, Inc.“We’re getting disclosures in, but they come with lessdetail,” adds Josh Mauldin, PhD, licensing manager with theUniversity of Virginia Licensing & Ventures Group. “You sitwith someone for hours and [then have to] say ‘Great, can youcontinued on page 180 65M put toward advancing promising agents through pre-clinical phaseHopkins collaborates withinvestment firm to de-riskearly-stage therapeuticsThe Johns Hopkins University and investment advisorDeerfield Management have launched a clever collaborationcalled Bluefield Innovations that will sponsor research intoearly-stage therapeutics then license the results, likely to pharmaceutical companies, or spin out start-ups built on the discoveries made by researchers at Johns Hopkins Medicine andthe Johns Hopkins Drug Discovery Program.From JHU’s perspective, the arrangement provides a fullyfunded and professionally supported pathway for faculty tosee the fruits of their labor realized -- and it ensures nicefinancial benefits for the school in the form of higher valuedeals for more mature assets.continued on page 183Technology Transfer Tacticsu Vol. 11, No. 12 (pp 177-192) December 2017uwww.TechTransferCentral.com
Attorney: Don’t put toomuch faith in sovereignimmunitySovereign immunity can add to the value ofpatents owned by state universities, but it is notan ironclad defense against patent challenges -at least not until we hear more from the courts,an attorney cautions.Go ahead and promote sovereign immunityas an extra bit of insurance to potential licenseesif you want, but don’t make promises that thepatent is safe from legal action, says PaulaEstrada de Martin, PhD, JD, a life sciences andbiotechnology patent attorney with the law firmof Baker Donelson in New Orleans.The issue of sovereign immunity has beentested three times in 2017, in cases involving theUniversity of Florida, the University ofMaryland, and the University of Minnesota. Ineach case, the Patent Trial and Appeal Boardruled that these state schools were immune fromIPR proceedings challenging their patents basedon the 11th Amendment of the U.S. Constitution.The cases are now giving rise to cases that pushthe boundaries of sovereign immunity, includingthe sale of patents to American Indian tribes,who then license the patents back to large pharma companies for cash and protect the companies from IPR challenges via their sovereignimmunity. The first such case involves IPRs filedby Mylan Pharmaceuticals against six patentsthat were owned by Allergan at the time of thefiling. In September Allergan assigned thepatents to the Saint Regis Mohawk Tribe, whichgranted back to Allergan an exclusive limitedfield-of-use license and then notified the Boardthat it was the new owner of the patents. A weeklater the Saint Regis Tribe filed a motion to dismiss based on tribal sovereign immunity.Another case with more direct impact onuniversities also appears to be stretching the limits of a sovereign immunity defense, Estrada deMartin says. Unlike the other cases involving IPRchallenges, Ali v. Carnegie Institution ofWashington pits a researcher who claims he wasimproperly omitted as a co-inventor seekingdamages. The suit has been dismissed by thecourts thus far on sovereign immunity grounds,but the researcher has petitioned to the U.S.Supreme Court.The Ali dispute involves a graduate student’s work at the University of Massachusettsregarding mRNA inhibitors. The researcher heworked with, Craig Mello, was awarded theNobel Prize along with a colleague fromCarnegie, and several related patents are ownedjointly by the universities.The graduate student was not listed as aninventor and sued the schools. A court dismissedthe University of Massachusetts on grounds ofsovereign immunity, and Carnegie was also dismissed because the University of Massachusettswas deemed an indispensable party to the litigation. The plaintiff is petitioning the SupremeCourt, but Estrada de Martin says sovereignimmunity is “a smokescreen” for the real issuesin the case, and it is not the right test case for thequestion of state schools being immune frompatent challenges.Pushing the envelopeSome tech transfer officials look at the Alicase and go too far in their conclusions aboutwhat it means for patents at state universities,she says. Universities would be better off focuscontinued on page 186Technology Transfer Tactics (ISSN 1940-1663) is published monthly by 2Market Information, Inc., 1992 Westminster Way, Atlanta, GA 30307 USATelephone: (239) 263-0605 Fax: (404) 381-1354 E-mail: ttc@techtransfercentral.com Website: www.techtransfercentral.comPOSTMASTER: Send address changes to Technology Transfer Tactics, 1992 Westminster Way, Atlanta, GA 30307 USA.CEO and Publisher: David Schwartz Marketing Director: Connie Austin Graphics and Web: Jason NorrisSubscriber Services: Valeasia Walker Events and Distance Learning: Debi MelilloTMSubscription rates: USA, USA possessions and Canada, one year (12 issues): 697. Other international subscriptions: 747. Back issues: 65 each.Technology Transfer Tactics is completely independent, and not connected with or controlled by any government agency, organization or society, consultancy,contractor, or vendor. Opinions expressed are not necessarily those of this publication. Mention of products or services does not constitute endorsement.Clinical, legal, tax, and other forms of professional advice are offered for general guidance only; competent counsel should be sought for specific situations.TMNOTICE: 2017 2Market Information, Inc. The entire contents of this, and every, issue of Technology Transfer Tactics are protected by Copyright, worldwide. All rights reserved. Reproduction or further distribution by any means, beyond the paid subscriber, is strictly forbidden without the written consent of thePublisher. This prohibition includes photocopying and digital, electronic and/or Web distribution, dissemination, storage or retrieval. Report violations in confidence; a 10,000 reward is offered for information resulting in a successful prosecution. Economical rates for bulk print or electronic subscriptions areavailable on request. Technology Transfer Tactics is a trademark of 2Market Information, Inc.TMTM178TECHNOLOGY TRANSFER TACTICSDecember 2017
TTOs struggle to keep up, citing staffing woesCreative solutions found forimproving reportingbetween IIA participantsIn any organization where resources are limited, choices must be continually made on theallocation of those resources. In TTOs, shortagesof time and staffing are common, and lack ofeither or both can cause some processes to fall bythe wayside -- or at least to get less attentionthan they might deserve.For many universities one of those areas isinter-institutional agreements, or IIAs, which areemployed when two institutions collaborate on asingle invention. Observers agree that mostschools do not provide adequate reporting orcommunication when operating under IIAs.“I’ve noticed that universities are not good atreporting patents as required by IIAs (my officeis just as guilty),” noted Gregg Banninger, patentcoordinator at the University of Illinois UrbanaChampaign, as he launched an AUTM listserv onthe topic. As he explains to TTT: “I think most ofus know that it’s an issue, but it’s such a low priority because we’re so overwhelmed trying to dopatents, licenses, and so on, and most of us areunder-staffed.”“I think that’s a true statement,” saysPatricia A. Reineke, RTTP, paralegal contractsadministrator in the Office of TechnologyLicensing, University of Florida.“We have this same issue when we are theco-owner and not leading prosecution and licensing, and I’m sure almost every TTO does aswell,” added Julie S. Kelley, business managerin Augusta (GA) University’s Office ofInnovation Commercialization, in responding theBanninger’s post.“I would say within our organization we’vebeen fairly lax in requesting reports from otherinstitutions,” concedes Rachael Widener, SeniorLicensing Manager with the University ofGeorgia’s Innovation Gateway. “We are copiedgenerally with all patent correspondence fromour attorneys so I feel we have knowledge ofwhat’s happening with the patents, but eventhough in the agreement it may ask for reportsevery year we’ve not been particular about that.”This is not to say that universities are simplyaccepting these shortcomings. “My job is to makeDecember 2017sure it’s all up to speed,” Banninger tells TTT.“I’ve been in tech, on the patent side, at a lawfirm, and I always like to have the other person’snumber. It will take work to get it in place and Iknow it’s a low priority for a lot of TTOs, butwe’re looking for ways where the docketing person will know who to send information to, aswell as the [reference] numbers.”And Banninger is not alone. A number ofuniversities have developed processes toimprove communication in this area -- and theyhave good reason to do so.Not without consequencesCommunication lacking in sufficient detailcan have repercussions ranging from annoyanceto excess cost -- in terms of time if not money.“One thing I get annoyed at with other universities is they have none of our numbers -- andsometimes the patent is not even reported,” saysBanninger, who manages the patent portfolio atUrbana-Champaign. “I’ve gotten invoices withno reference to the patent number, and no reference number, so we can’t pay them.”The most common problem, he continues, isthat this lack of proper communication createsmore work for the other party. Lack of notification on some patent and office actions “could beproblematic if a partner decides not to move forward, and maybe our inventor feels stronglyabout it,” Banninger points out.Kelly says that reporting progress on marketing/licensing to co-owners is the first area tobe neglected, and that reporting patent activity isnot far behind. “That’s where we really fallbehind; everything else on our plate is peoplesaying ‘I’ve got to have this,’” she says. “Sincethis is not something the university comes to askabout, it’s the first thing that falls off.”“There are a couple of reasons why it’s aslower process,” adds Reineke. In some cases,she says, there is a lead patent holder who thensigns an IIA with a co-patent owner. “You havenow signed up to share costs, but it’s a naturaltendency to say ‘I’ll get that done later,’” sheobserves. “So [if you are the lead and] not payingattention, you’re carrying all the costs as a university so you lose money.”The other impact, she continues, is if you getall the way to a license and forget the other institution. It will surface eventually, and then “it’sWWW.TECHTRANSFERCENTRAL.COMcontinued on page 187179
Research tools continued from p. 177get me something in writing?’”One of the strategies universities areemploying in an attempt to meet this challenge isthe establishment of partnerships with third parties, who in turn pay for interns to help in theprocess. For example, Gama has established anon-exclusive partnership with Kerafast, aprovider of RTs for the academic and industrialcommunity.Kerafast, based in Boston, works with morethan 150 institutions globally, offering theirreagents and other research tools through anonline platform. The platform allows the materials to be “quickly accessed without needing togo through the traditional Material TransferAgreement process,” the company says on itswebsite. Kerafast does all the work involved inselling and delivering materials and pays royalties to its university partners based on thosesales. “We therefore help providing labs savetime and resources, while generating extrafunding for further research. Procuring scientists can more easily discover and access uniquereagents that are often unavailable elsewhere,while also funding the work of otherresearchers,” the company says.“I’ve been here 15 to 16 years and alwayswanted to implement a robust research toolslicensing program -- especially antibodies,” saysGama. “It was very difficult for us to do that inthe context of being a manager who already hada portfolio of technologies.” In addition, hepoints out, the identification of new tools is notsimple. “You look into the literature, compare itwith our databases -- it’s very convoluted,” hesays.Gama knew the school had potentially thousands of tools, but he needed more informationon them. Kerafast, as part of its onboardingprocess, provided interns to help dig into theschool’s research tool portfolio. “Also, they’re alittle different -- more oriented to the creators ofmaterials,” he explains.In addition, Gama says, “They create pagesbriefly describing the research activities of theinvestigators that have materials with them -they’re unique that way. This gives us the opportunity not only to license but to make the materials and UGA research known beyond the academic environment.” (The page Kerafast created forUGA can be found at: of-georgia.UGA’s marketing efforts also involve the creationof technology catalogs. The one for research tools canbe found at: https://uga.flintbox.com/public/project/31547/ “It was our first try at one of these, and weare now creating a new design for future editions,”says Gama.)There is no cost to UGA, he adds. “Theylicense the materials from us and then sell thosematerials,” he explains.Another outsourcing optionAt the University of Iowa ResearchFoundation, Jane Garrity, PhD, associate directorfor outreach and engagement, is in the process ofsetting up a partnership with Ximbio, a non-profit that markets, distributes, and sublicensesreagents like antibodies, cell lines, and mousemodels.“When we started talking to them we hadbeen looking at ways to streamline and outsource some of our material licensing,” Garrityshares. “First off, in terms of the workload forlicensing associates these are typically lowvalue deals, but they take time to negotiate. Oneantibody at a time is not necessarily the mostefficient [way to go].”In addition, she continues, the partnershipoffers a way to improve service to inventors.“Lots of times when we get potential licenseeswe have to reach out to the inventor to get all thetechnical information on an antibody or cell line;there is a lot of negotiation and it can be a prettybig time commitment for them,” says Garrity.“Hopefully, we’ll get to the point where the disclosure material has all the information rightaway -- so it’s sent out once and distributed bysomeone else later on.”Garrity says she also looked at Kerafastand other companies before choosing Ximbio.“First, they seemed to have a pretty broadrange of things they worked with -- like mousemodels, bacteria, plasmids,” she says. “It’s notlike working with one company for one material. The other thing that appealed to us is thatthey actually will sublicense the materials sothey can work with another company that’sselling antibodies.” Some schools might preferseparate licenses, she notes, “but we like thetime savings.”Under the agreement, she continues, XimbioTECHNOLOGY TRANSFER TACTICScontinued on page 181December 2017
Research tools continued from p. 180would have the right to either sell materialsdirectly or sublicense them, “and we get a prettygenerous share,” Garrity adds. She declined toreveal the actual figures.Interns overcome barriersAt UGA, the work with interns began withmonoclonal antibodies “for a very simple reason-- we have one of the largest monoclonal antibody facilities in the country,” says Gama, who isquick to note this is a collective effort involvingmany if not all of his colleagues, as most haveRTs in their portfolios.The school had in storage about 2,000 lines -some developed by UGA investigators, whileothers were not. “The interns looked at thosedeveloped by us and got background information on each and every line,” says Gama. Theprocess took about three years to complete,yielding information on 300 to 350 lines.Using the interns to do the heavy liftingovercame a common barrier to more extensivelicensing of research tools.“There is a very good disclose rate wheninvestigators perceive a high value, but tools typically show a low value per material,” Gamanotes. “When we approach them and ask forinformation that may be four, five, or six yearsold and they don’t perceive great value, responsemay be a little more delayed. So, we actually hadto be proactive -- find the information we couldfind, and then we created the disclosure documentation ourselves and [only] asked them toverify if the [forms] were correct.” In a way “it islike an inverted inventor outreach -- we createthe disclosure and then engage the inventors,”Gama adds.As part of this process, new, shorter disclosure forms were designed. “We actually creatednew ones for each category of RT -- antibodies,plasmids, and so on -- about six or seven in all,”says Gama. The original form, he says, had threeor four pages, while the new one has only one.(See the example, page 182.)Interns are also a big part of the researchtools initiative at Iowa, where they “essentiallywork as scouts for research reagents,” saysGarrity. “They go to labs on campus, and theytrack well with the licensing associates. They findout what people have in their freezers, and helpDecember 2017researchers do the disclosures.”Garrity also plans to have a new, streamlinedform. It will ask basic question such as, “What isthis antibody?” “How much do you have?”instead of many other questions typically foundon disclosures “that are not relevant for them,”she explains, adding that the students also helpget materials prepared and shipped out.Taking a different tackMauldin says he has basically never utilizedinterns specifically dedicated to this type ofwork. “With most of our licensing interns theyare PhD students, so they are skilled to do thiswithout very much training; we do not need toexplain what we’re looking for,” he says. “If wehad an intern from our business school or lawschool we would just not put them on this sort oftask.”His approach, rather, involves the followingrecommendations: Combing through PubMed for manuscriptswith the university’s affiliation and reading theMaterials & Methods sections of those paperssearching for new materials; Monitoring MTA requests for outgoingmaterials to identify in-demand reagents; or If your university has core facilities forproducing antibodies (hybridomas and/or polyclonals) and/or genetically engineering mice (orother model organisms), ask the managers ofthose facilities which faculty members have usedtheir services over the past few years.“Mining the literature is pretty common,”says Mauldin. “I had one colleague who had analert set up in PubMed looking for any paperscoming out with affiliation with UVA. It’s fairlyeasy to do if you go to the website.” If your interest is more specific to a particular research topic,he adds, you can set up alerts on your protein ofinterest.UVA has some partners at reagent companies, he continues. “We get e-mails saying, ‘Wesee this was made by UVA, can we get it?’” hesays. “It probably takes a fair bit of combing.”UVA’s antibody core opened in 1982, and“I’ve seen agreements going back to ‘91, ‘92 orearlier,” says Mauldin. “We’ve been tapped intointernational antibody generation for severaldecades. Now we’re finding a lot of new stuffsince we’ve been actively monitoring it.”WWW.TECHTRANSFERCENTRAL.COMcontinued on page 183181
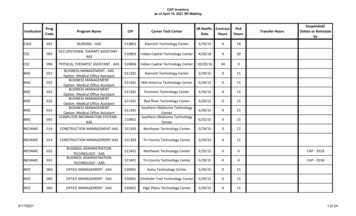
Figure 1Source: Innovation Gateway, University of Georgia Office of Research182TECHNOLOGY TRANSFER TACTICSDecember 2017
Research tools continued from p. 181Benefits beyond revenueIt’s no secret that in general these tools do notgenerate a great deal of revenue, but that doesn’tmean increased disclosures and licensing do nothave their benefits. “Being more hands on andproactive, as well as having some ‘dedicatedhelp,’ proved to be the way to go,” Gama insists.“Over the period of just about four years, we havemanaged to build a portfolio of nearly 500 researchtools (antibodies, cell cultures, GMO bacteria/yeast,exotic chemicals, etc.) and license nearly all of them.We probably have executed well over 100 licenseagreements in this area, with five to 10 companies,during this period.” (Most of these are listed athttps://uga.flintbox.com/public/ project/31547/InnovationGateway.)UGA has a Monoclonal Facility and aBiofermentation and Expression Facility, he adds.“This helps a lot to have identifiable materials aswell as create leads for services and sales agreements in connection with licenses,” says Gama.He says that while these licenses have created “some” revenue, what he’s really after isrecognition of research activity, most importantlythe making of rare reagents that only UGA hasand which are available to the research community. “We have a rich history at UGA of RTs,” henotes. “Some those from the 70’s, 80’s, 90’s, suchas luciferase from Renilla, Aequorin cDNA and theBradford Assay, generate a lot of income. Maybesome of the new ones will generate a lot ofincome, [too].”There is an additional benefit, he continues,which involves MTAs. “Typically these [materials] are sent out through MTAs; the process isoften protracted and can be antagonistic,” saysGama. “By entering into license agreements andmaking them commercially available, it reducesthe need for those materials to be obtainedthrough MTAs. Of course, we still provide MTAsfor researchers who ask.”Garrity agrees. “We use it as a way to reducethe burden of MTAs,” she notes. “We do haveoutgoing MTAs that go through our office -mostly research reagents or bio-specimens. Butthe main [benefit] is for researchers; sometimesMTAs can take a while to negotiate if you look tocollaborate with someone, especially in industry.Plus, if you have material that’s very popularDecember 2017they have to prepare it, ship it out, and do testing. If we can get someone else handling it andall the shipping and negotiation, researchers willnot [need to] spend time on it, and licensingfolks and monetary folks can handle the highervalue or more complex deals. Also, if this startsto move more smoothly we’ll have more materialdisclosures and reagent disclosures, which helpsbuild up the portfolio.”Most of the benefit, she continues, falls to theresearchers. “They’re able to get their materialsout more broadly,” Garrity says. “Also, it provides a service to the scientific community. Andit is a small revenue stream for those labs; themajority of that income does go to researchers’labs, and some of them certainly appreciate it.”Looking ahead, Gama says, “we want toengage the faculty into the process a little better,making it simpler for them and engage them inthe beginning. It’s not necessarily the disclosureform for us, but making us aware they havethings in the lab they’d like to license. That hashappened.”This applies not only to materials but also toprocesses, he continues. One consequence of thatapproach, for example, occurs when enzymes areinvolved and the licensee requires production ofthem to sell. “As part of our program we’reencouraging the establishment of a service andsales partnership between the company and theinvestigator,” says Gama. “Once they run out,they could ask the investigator for more; whenthis is paid, it creates more revenue. This isanother goal of licensing; to produce researchrevenue for the investigator.”Contact Gama at 706-583-8088 or GJG@uga.edu;Garrity at 319-335-5795 or jane-garrity@uiowa.edu;and Mauldin at 434-243-7183; e-mail:josh@uvapf.org. uJohns Hopkins continued from p. 177Bluefield Innovations, the pair say, will catalyze the development of early-stage therapeutics with funding from Deerfield -- up to 65million over five years is the initial financialcommitment. Dave Greenwald, director of business development at Johns Hopkins TechnologyVentures, reports that a joint steering committee,with three members from each entity, will identi-WWW.TECHTRANSFERCENTRAL.COMcontinued on page 184183
Johns Hopkins continued from p. 183fy the research Bluefield will fund through preclinical development -- basic research, proof ofconcept studies, target selection andInvestigational New Drug-enabling studies.Anything developed under the Bluefieldbanner will be licensed to third parties or spunout, he adds, with Deerfield potentially investing in start-ups around technology it helped toadvance. He notes that the combination ofHopkins’ discovery expertise and Deerfield’sfinancial support enables the entity to minimizethe financial and developmental risk usuallyassociated with early-stage therapeuticsresearch.Good relationship, good timingPartnerships like that don’t happen everyday, and this one came about because of a longterm relationship the two already enjoyed -- andexcellent timing.“The deal with Deerfield really startedbecause we have a very good existing relationship,” Greenwald says, pointing out that the firmfunded two Hopkins start-ups -- BladeTherapeutics and Graybug Vision Inc. “Both onthose start-ups, and just professionally, we had agood relationship going into this collaboration,”he adds. “Both institutions were looking to dosomething like this, so it came together quite naturally. It was a combination of a good existingrelationship and good timing. We’re constantlylooking to do creative deals, and Deerfield waslooking to engage more with academia.”It’s not Deerfield’s first rodeo. The companyhas been an increasingly active player in academic partnerships with a defined investment pathway. Their recent deals include: The 550 million Deerfield HealthcareInnovations Fund LP “invests in therapeutic interventions in genetic diseases, cancer and orphandiseases.” Investors include “leading universitieswith cutting-edge research, such as PrincetonUniversity and Northwestern University.” Deerfield and Bay City Capital, joined byTakeda Pharmaceutical Company Ltd., launched adrug discovery company called Bridge Medicines;partners include The Rockefeller University andWeill Cornell Medicine. It builds on the work ofthe Tri-Institutional Therapeutics DiscoveryInstitute, launched in 2013, that funds some 50184early-stage projects with principal investigatorsand Takeda scientists collaborating to demonstratetherapeutic viability. Those projects then can graduate to Bridge to be “professionally managed in aventure capital setting.” PIs “who choose tobecome engaged could continue on as equitystakeholders.” The
178 TECHNOLOGY TRANSFER TACTICS December 2017 Technology Transfer Tactics TM (ISSN 1940-1663) is published monthly by 2Market Information, Inc., 1992 Westminster Way, Atlanta, GA 30307 USA Telephone: (239) 263-0605 Fax: (404) 381-1354 E-mail: ttc@techtransfercentral.com Website: www.techtransfercentral.com