Transcription
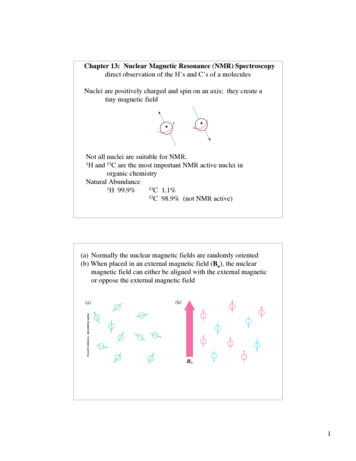
Chapter 13: Nuclear Magnetic Resonance (NMR) Spectroscopydirect observation of the H’s and C’s of a moleculesNuclei are positively charged and spin on an axis; they create atiny magnetic field Not all nuclei are suitable for NMR.1H and 13C are the most important NMR active nuclei inorganic chemistryNatural Abundance1H 99.9%13C 1.1%12C 98.9% (not NMR active)(a) Normally the nuclear magnetic fields are randomly oriented(b) When placed in an external magnetic field (Bo), the nuclearmagnetic field can either be aligned with the external magneticor oppose the external magnetic field1
The energy difference between aligned and opposed to the externalmagnetic field (Bo) is generally small and is dependant upon BoApplied EM radiation (radio waves) causes the spin to flip and thenuclei are said to be in resonance with BoDE h nDE Note that h2pBo external magnetic field strengthg gyromagnetic ratio1H 26,75213C 6.7is a constant and is sometimes denoted as hgBo h2pNMR Active Nuclei: nuclear spin quantum number (I)atomic mass and atomic numberNumber of spin states 2I 1 (number of possible energy levels)Even mass nuclei that have even number of neutron have I 0(NMR inactive)Even mass nuclei that have odd number of neutrons have aninteger spin quantum number (I 1, 2, 3, etc)Odd mass nuclei have half-integer spin quantum number(I 1/2, 3/2, 5/2, etc)I 1/2: 1H, 13C, 19F, 31PI 1: 2H, 14NI 3/2: 15NI 0: 12C, 16O2
Continuous wave (CW) NMRPulsed (FT) NMRDifferent nuclei absorb EM radiation at different wavelength (energyrequired to bring about resonance)Nuclei of a given type, will resonate at different energies depending ontheir chemical and electronic environment.The position (chemical shift, d) and pattern (splitting or multiplicity)of the NMR signals gives important information about thechemical environment of the nucleiH OH HH C C O C C HHH H3
Chemical shift: the exact field strength (in ppm) of a nuclei comesinto resonance relative to a reference standard (TMSElectron clouds “shield” nuclei from the external magnetic fieldcausing then to absorb at slightly higher energyShielding: influence of neighboring functional groups on the electronicstructure around a nuclei and consequently the chemical shiftof their resonance.CH3Tetramethylsilane (TMS);OH3C C OCH2CH3H3C Si CH3CH3Reference standard d 0for 1H NMRVertical scale intensity of the signalHorizontal scale chemical shift (d), dependent upon the field strengthof the external magnetic field; for 1H, d is usually from 1-10 ppmn- nTMSchemical shift in Hzd nooperating frequency in MHz14,100 gauss: 60 MHz for 1H (60 million hertz) ppm 60 Hz15 MHz for 13C140,000 gauss: 600 MHz for 1Hppm 600 Hz150 MHz for 13C4
Equivalence: chemically and magnetically equivalent nuclei resonateat the same energy and give a single signal or patternH3CHC CH3CCH3Test of Equivalence:1. Do a mental substitution of the nuclei you are testing with anarbitrary label (your book uses X)2. Ask what is the relationship of the compounds with thearbitrary label3. If the labeled compounds are identical (or enantiomers), then theoriginal nuclei are chemically equivalent and will normallygive rise to a single resonance in the NMR spectraIf the labeled compounds are not identical (and not enantiomers),then the original nuclei are not chemically equivalent and cangive rise to different resonances in the NMR spectraH HH CCH3C CH3CCH3H HH CCH3C CH3CCH3H HH CCH3C CH3CCH3H HH CCH3C CH3CCH3Identical, so the protons are equivalentH3CCH3H3CC CH3CCH3H3CC CCH3H3CCH3H3CC CCH3H3CCH3H3CC CCH3H3CCH3C CCH3H3CCH3Identical, so the methyl groups are equivalent5
H3CHH3CC CH3CH3CHH3CCH3H3CHH3CH3CC CHC CC CCH3H3CCH3HC CCH3H3CHH3CH3CC CH3CH3CCH3HC CCH3H3CCH3These are geometricisomers (not identical andnot enantiomers). The threemethyl groups are thereforenot chemically equivalentand can give rise to differentresonancesHC CH3CCH3HHH HCH2CH3HH HH HHHCH2CH3HH6
H ClH ClH ClH3C C C CH3H3C C C CH3H3C C C CH3H HH HH HH ClH HH HH3C C C CH3H3CH HCH3CH3H3CH ClH ClH HH HH HCH3CH3CH3Homotopic: equivalentEnantiotopic: equivalentDiastereotopic: non-equivalentCyclohexane: two different types of protons, axial and equitorialHHHHHHHHHHHHHHHHHHHHHHHHThe chair-chair interconversion interchanges the axial and equatorialprotons and is a fast process at room temperature. NMR islike a camera with a slow shutter speed and a blurred image offast processes is observed.At room temperature the cyclohexane protons are observed as atime-average and appear as a single resonance. At -90 C thechair-chair interconversion is sufficiently slow that axial andequatorial are observed as two separate resonances.7
Typical 1H NMR chemical shifts rangesalso see Table 13.2 and 13.3 (pages 495-6)121110987654312H1ROCR2OHH NMR Shift RangesRCOR2NRCHPhOHCR2R C-H1211HX CR2 X F, Cl, Br, Iaromatics109876ROHsat. alkanesR-HOvinylRCO2HCR2X O, CR2NRCHOHHHO CR2OR2N-HCR2R2NHXCR2ArH0HC C543H210d (PPM)The influence of neighboring groups (deshielding) on 1H chemicalshifts is additive (to an extent)Shoolery’s additivity rules for predicting the chemical shift of protonsof the type:Y- or X C Hfor protons onX C Hsp3 carbons onlyd (ppm) 0.233 S siFuntional Group (X,Y)-Cl-Br-I-OH-ORsi (ppm)2.532.331.822.563.23Funtional Group 13si (ppm)R1.70OORONR2-CF3-CN1.551.591.141.708
d 2.0d 1.2d 4.1H OH HH C C O C C HHH H O-or-HH C C C O C HH HH HHd 0.233 1.70 0.47 2.43d 0.233 3.13 3.363d 0.233 3.13 0.47 3.833d 0.233 1.70 1.933Integration of 1H NMR resonancesThe area under an NMR resonance is proportional to the number ofnuclei that give rise to that resonance.d 1.2d 3.66integral2The relative area under the resonances at d 3.6 and 1.2 is 1:3The integral is superimposed over the spectrum as a “stair-step” line.The height of each “step” is proportional to the area under the resonance.9
d 1.6d 3.44.53H OH Hd 2.03HH C C O C C HHd 1.23HH Hd 4.12HSpin-Spin Coupling (splitting)protons on adjacent carbons will interact and “split” eachothers resonances into multiple peaks (multiplets)n 1 rule: equivalent protons that have n equivalent protons onthe adjacent carbon will be “split” into n 1 peaks.H OH Hd 2.03HH C C O C C HHd 1.23HH Hd 4.12HResonances always split each other. The resonance at d 4.1 splitsthe resonance at d 1.2, therefore the resonance at d 1.2 must splitthe resonance at d 4.2.10
The multiplicity is defined by the number of peaks and the patternd 1.7d 3.4H HH C C BrH H-CH3--CH2-1:3:3:11:2:1One proton on an adjacent carbon will split a proton into a doublet (d),two peaks of 1:1 relative intensityTwo proton on an adjacent carbon will split a proton into a triplet (t),three peaks of 1:2:1 relative intensityThree proton on an adjacent carbon will split a proton into a quartet (q),four peaks of 1:3:3:1 relative intensityEquivalent protons do not show spin-spin couplingH HHBr C C BrH C BrH HHR2Singlet (s)R111
The resonance of a proton with n equivalent protons on the adjacentcarbon will be “split” into n 1 peaks with a coupling constant J.Coupling constant: distance between peaks of a split pattern; expressedin Hz. Protons coupled to each other have the same couplingconstant J.H HH C C BrH H12
More complex spin-spin coupling: non equivalent protons will coupleindependently.H2 splits H3 into a doublet with coupling constant J2-3H2 splits H1 into a doublet with coupling constant J1-2H1 splits H2 into a doublet; H3 splits H2 into a doublet(doublet of doublets) with coupling constants J1-2 and J2-3.13
Summary of 1H-1H spin-spin coupling chemically equivalent protons do not exhibit spin-spin coupling toeach other. the resonance of a proton that has n equivalent protons on theadjacent carbon is split into n 1 peaks (multiplicity) with acoupling constant J. protons that are coupled to each other have the same couplingconstant non-equivalent protons will split a common proton independently.complex coupling.Spin-spin coupling is normally observed between nuclei that areone, two and three bonds away. Four-bond coupling canbe observed in certain situations but is not common.Summary of 1H-NMR Spectroscopy the number of proton resonances equals the number ofnon-equivalent protons the chemical shift (d, ppm) of a proton is diagnostic of the chemicalenvironment (shielding and deshilding) Integration: number of equivalent protons giving rise to a resonance spin-spin coupling is dependent upon the number of equivalentprotons on the adjacent carbon14
13CNMR Spectroscopy:Natural Abundance1H 99.9% (I 1/2)O12C13C1.1 %1.1 %1.1 %Bo external magnetic field strengthg gyromagnetic ratio1H 26,75213C 6.7DE gBo h2p13C98.9% (I 0)1.1% (I 1/2)H3C C OCH3is a much less sensitive nuclei than 1H for NMR spectroscopyNew techniques (hardware and software) has made 13C NMR routine Pulsed NMR techniques (FT or time domain NMR) Signal averaging (improved signal to noise)Pulsed NMR Techniques:ZZEM pulse “tips” themagnetization 90 into the XY-planeXXThe magnetization isdetected in the X-axisYYThe magnetization precesses in theXY-plane at the Larmor frequency ofthe nuclei, which is directly related tothe chemical shift (d) of the nucleiZrelaxation(recovery)XYThe magnetization will relax (recover) back tothe Z-axis. As the magnetization precessesin XY-plane, it “spirals” back to the Z-axis.Animation: http://www.nmr.ethz.ch/education/PCV/anim/puls evol.htmlhttp://www.nmr.ucdavis.edu/BCM230 F2001/Flash/PresentationSecondWeek.swf15
Free Induction Decay (FID)- time domain NMRtimeIn pulse (FT) NMR, all nuclei are tipped at the same time and theFID’s are superimposed.Fourier Transform (FT) deconvolutes all of the FID’s and gives anNMR spectra.Signal averaging: pulsed NMR allows for many FID’s (NMR spectra)to be accumulated over time. These FID’s are added togetherand averaged. Signals (resonances) build up while the “noise”is random and cancels out during the averaging.Enhanced signal to noise ratio and allows for NMR spectra tobe collected on insensitive nuclei such as 13C and small samples.Chemical shifts give an idea of the chemical and electronic environmentof the 13C nuclei due to shielding and deshielding effectsrange: 0 - 220 ppm from TMS13CNMR spectra will give a map of the carbon framework. Thenumber of resonances equales the number of non-equivalentcarbons.16
Signal-Averaging13C-spectra of CH CH CH CH CH OH32222after one scanafter 200 scansChemical Shift Range of 13C22020013180160140120100806040200-20R3C-BrC NMR Shift RangesR3C-FR3C-ClR3C-IR2N-CR3R3C 180160Ar-CR3saturatedalkanesketones & esters, amidesaldehydes & acids220CR3140120100806040200-20d (PPM)Note the carbonyl range17
1H-13C13Cspin-spin coupling: spin-spin coupling tells how many protonsare attached to the 13C nuclei. (i.e., primary, secondary tertiaryor quaternary carbon)spectra are usually collected with the 1H-13C coupling “turnedoff” (broad band decoupled). In this mode all 13C resonancesappear as singlets.DEPT spectra (Distortionless Enhancement by Polarization Transfer)a modern 13C NMR spectra that allows you to determine thenumber of attached hydrogens.Run: broad-band decoupled spectraDEPT-90: only CH’s show upDEPT-135: CH’s and CH3’s give positive resonancesCH2’s give negative resonances18
876543238 7Broad-band 2Solving Combined Spectra Problems:Mass Spectra:Molecular FormulaNitrogen Rule Æ # of nitrogen atoms in the moleculeM 1 peak Æ # of carbonsDegrees of Unsaturation: # of rings and/or p-bondsInfrared Spectra:Functional GroupsC OC CC C1H NMR:Chemical Shift (d) Æ chemical environment of the H'sIntegration Æ # of H's giving rise to the resonanceSpin-Spin Coupling (multiplicity) Æ # of non-equivalent H's on theadjacent carbons (vicinal coupling).Shoolery's Rules: finial check on the structure assignment by 1H NMR13C NMR:# of resonances Æ symmetry of carbon frameworkType of CarbonylEach piece of evidence gives a fragment (puzzle piece) of the structure. Piece thepuzzle together to give a proposed structure. The proposed structure should beconsistent with all the evidence.19
Magnetic Resonance Imaging (MRI): uses the principles ofnuclear magnetic resonance to image tissueMRI normally used the magnetic resonance of protons on waterand very sophisticated computer methods to obtain images.Other nuclei within the tissue can also be used (31P) or aimaging (contrast) agent can be administeredNormal25 years oldNormal86 years oldAlzheimer’s Disease78 years old20
organic chemistry Natural Abundance 1H 99.9% 13C 1.1% 12C 98.9% (not NMR active) (a)Normally the nuclear magnetic fields are randomly oriented (b) When placed in an external magnetic field (Bo), the nuclear magnetic field can either be aligned with the external magnetic or oppose the external magnetic field