Transcription
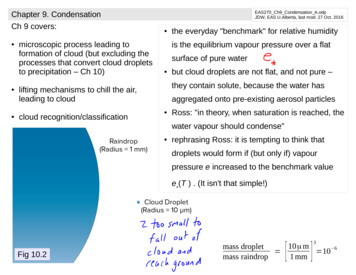
Chapter 9. CondensationCh 9 covers: microscopic process leading toformation of cloud (but excluding theprocesses that convert cloud dropletsto precipitation – Ch 10)EAS270 Ch9 Condensation A.odpJDW, EAS U.Alberta, last mod. 27 Oct. 2016 is the equilibrium vapour pressure over a flatsurface of pure water but cloud droplets are not flat, and not pure –they contain solute, because the water haslifting mechanisms to chill the air,leading to cloudcloud recognition/classificationthe everyday "benchmark" for relative humidityaggregated onto pre-existing aerosol particles Ross: "in theory, when saturation is reached, thewater vapour should condense" rephrasing Ross: it is tempting to think thatdroplets would form if (but only if) vapourpressure e increased to the benchmark valuee*(T ) . (It isn't that simple!)Fig 10.2[]310μ mmass droplet 6 10mass raindrop1 mm
Sec 9.1 Homogeneous vs. hetereogeneous nucleation; curvature effect relative humidity in clouds rarely goesCurvature effect: supersaturation required toprevent evaporation ofa droplet of pure waterof the given radiusabove about 101% 2/10a considerably higher degree ofsupersaturation would be required toprevent evaporation of sub-micron sizedwater droplets "homogeneous nucleation," i.e.formation of pure water droplets bycollision and aggregation of vapourFig 9.1molecules, is NOT the mechanism for"Kelvin curve" gives thesupersaturation requiredto prevent evaporation ofa pure droplet of givenradiuscreating cloud droplets hetereogeneous nucleation: watermolecules condense onto aerosols if water deposits onto a wettable aerosolcapable of acting as cloud condensationwith radius 0.2 μm, it forms a film over thenuclei (CCN)surface; and can grow if RH exceeds aboutto act as CCN, aerosols must be100.5%"hydrophilic" (wettable) initial radius of wet CCN aerosol size once a droplet reaches about 1 μm radius itsequilib. v.p. is effectively that of a plane sfc
Sec 9.1 Solute effect – hygroscopic CCN hygroscopic CCN are aerosols thatdissolve in the water that depositsonto them a small mass of (e.g.) salt dissolvedin a droplet permits that droplet to bein equilibrium in sub-saturated air.the solute effect. Water will condenseonto salt aerosols with RH as low as70-80% 3/10Blue: curvature effecton pure dropletOther colours:solute effect pluscurvature effect: RHneeded to assureequilibrium of adroplet of impurewater of the givenradiusRH inhypotheticalcloud 100.2%in a cloud whose RH is 100.2% adroplet formed on a salt aerosol(NaCl) of mass 10-19 kg will grow untilit reaches equilibrium with a radiusFig 9.3just over 0.1 μm. forming a haze.But in that same cloud a salt aerosol(NaCl) of mass 10-18 kg will growwithout limit (be "activated") if cloudRH is sustained (despite droplet growth)At equal temperature, the equil. v.p. over a plane surface of asolution is lower than that over a plane surface of pure water
Ch 9. Condensation – relative growth rates of activated droplets 4/10considering the population ofactivated droplets (those able to grow"Kelvin curve"Fig 9.3without limit, i.e. those for which thepeak of the Kohler curve lies belowcloud RH), the smaller ones growfaster than the big ones** – tendingto give the cloud a distribution ofsame-sized cloud droplets RH inhypotheticalcloud 100.2%this population "competes" for water,the finite supply of which ultimatelylimits droplet size growth by diffusion– indeed growth of the droplets (by"Kohler curves"diffusion) tends to deplete the cloudair of water vapour for the same size droplets, higher RHneeded to grow those formed oninsoluble CCN than those formed onsoluble CCN**this is not obvious; the proof is laborious
Ch 9. Condensation – differing droplet populations in oceanic and continental air the bigger a droplet, the slower itsgrowth rate (not proven) 5/10Fig 9.4so smaller droplets "catch up" withbiggerDiffusion of vapour to thedroplets cannot growthem much larger thanabout 10 micronsand together, this population ofdroplets is soaking up vapour fromthe cloud airlimiting the ultimate size of thedroplets to a nominal 10 micronsactual size depends on number ofCCN available, and moisture supplygiven the same initial RH, anairmass with a higher count of CCNwill produce more, but smaller, clouddropletsaerosols most favourable for cloudformation are large, wettable andhighly soluble – e.g. salt aerosolsfrom evaporated ocean spray).Continents?. dustier, but dust is nothygroscopicUnits on the vertical axisshould be cm-3 μm-1Continentalair: morenumerous– butsmaller –clouddropletsOceanic airFig 9.5
Sec 9.2 Nucleation of droplets to form ice crystals – homogeneous nucleation6/10"Pure water does not necessarily freeze at 0oC. In fact, temperatures must drop to about-40oC before water drpolets will spontaneously freeze to form ice crystals. larger dropletswill freeze at slightly warmer temperature than will smaller droplets"Temperature 100-10-20-30largestdropsfrozen“ice embryos” formspontaneously but aremostly destroyed bythermal agitation of thecrystal lattice – exceptat very low temperature-40alldropsfrozenbigger droplets willhost more embryos –better chance of anembryo that survivesand growsThe smaller thevolume of asample of purewater, the lowerthe temperature atwhich it freezes
Sec 9.2. Hetereogeneous nucleation7/10 ice crystals may form in subfreezing air on "ice nuclei" of several types particles are effective ice nuclei if their crystal structure resembles that of ice more effective at lower temperatures ice nuclei generally rarer than condensation nucleiDue to the rarity of ice nuclei,the lower regions of a coldcloud (i.e. below about the levelof the -40oC isotherm) contain few ice crystals many supercooled dropletsFig 9.6
Sec 9.3 Mechanisms producing lift that can result in parcel cooling and cloudFig 9.7a) buoyant forcingb) orographic forcingc) low level convergence due tocross-isobar winds (weathersystem cloud)d) frontal lifte) divergence aloft8/10
Ch 9. Forcing mechanism dictates the scale and organization of cloudHeight/width(aspect ratio)Fig 9.7a) sporadic, randomly distributed,small scale, vert. development maybe large or smallb) orographic forcing – linearorganization, tied to landscapec,e) weather system scale, long lived(cloud shield)d) frontal lift – linear organization9/10
Topics/concepts covered role of CCN; countervailing curvature and solute effectstendency to grow a population of equi-sized, small cloud droplets – thatby virtue of their competition for water vapour are size-limitedrole of ice nuclei, and the co-existence of liquid and frozen cloud particlesin cloud layers with temperatures in the range (roughly) 0oC to -40oCmechanisms that result in lift, potentially initiating cloudLenticular cloud (Figure 9.11a)caused when a stable air layeris forced to ascend as it blowsover the mountains – this istermed “orographic” cloud(because it is forced by theorography, i.e. topography)
Ch 9. Forcing mechanism dictates the scale and organization of cloud 9/10 Fig 9.7 a) sporadic, randomly distributed, small scale, vert. development may be large or small b) orographic forcing – linear organization, tied to landscape c,e) weather system scale, long l










