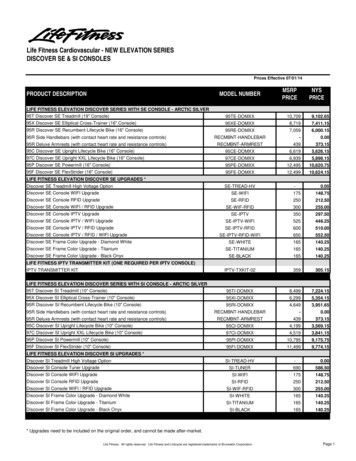
Transcription
TogetherWe DiscoverReaching Patients ThroughImmunology InnovationVYVGARTTM(efgartigimod alfa-fcab) FDA Approval CallDecember 17, 2021
Forward Looking StatementsThis presentation has been prepared by argenx se (“argenx” or the “company”) for informational purposes only and not for any other purpose. Nothingcontained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or the company or any director,employee, agent, or adviser of the company. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Thispresentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other dataabout our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates.!Safe Harbor: Certain statements contained in this presentation, other than present and historical facts and conditions independently verifiable at thedate hereof, may constitute forward-looking statements. Examples of such forward-looking statements include those regarding its statements related toits the therapeutic and commercial potential of VYVGART; the response to treatment paradigm, including rapid HCP adoption of VYVGART; theestimated number of gMG patients in the U.S.; infrastructure, access and path to bring VYVGART to patients; the expected effect of value-basedagreements with participating plans; expected pricing of VYVGART; the intended results of its strategy including global launch preparation; acceptance ofefgartigimod for review by PMDA and anticipated approval in first quarter of 2022; that MAA filed with EMA and validated, with anticipated approval insecond half of 2022; anticipated pathway for approval in China with NMPA by mid-2022; partnership agreement with Medison and anticipated filing inIsrael during first quarter of 2022; its pre-approval programs in Europe and Canada; ambition to be in 15 efgartigimod indications by 2025; its clinicaldevelopment and regulatory plans, including the timing and outcome of regulatory filings and approvals; the timing, progress and benefits of marketingand commercialization activities; and the expected size of the markets for VYVGART.2
VYVGARTApprovalTim Van HauwermeirenChief Executive Officer3
44
Where critical patient needmeets breakthrough scienceThat is where we redefine immunology5
VYVGART: Highlights of U.S.Prescribing InformationINDICATION STATEMENTVYVGART is a neonatal Fc receptor blocker indicated for the treatment of generalizedmyasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR)antibody positiveDOSING AND ADMINISTRATION Evaluate need to administer age-appropriate vaccines according to immunizationguidelines before initiation of new treatment cycleRecommended dosage is 10 mg/kg administered as an intravenous infusion over onehour once weekly for 4 weeksSubsequent treatment cycles to be administered based on clinical evaluationWARNINGS AND PRECAUTIONS Delay administration to patients with active infection. Monitor for signs andsymptoms of infection. If serious infection occurs, administer appropriate treatmentand consider withholding VYVGART until infection has resolvedAngioedema, dyspnea, and rash have occurred. If a hypersensitivity reaction occurs,discontinue the infusion and institute appropriate therapy6
KIMLiving with gMGGENERALIZEDMYASTHENIA GRAVISPatientExperience1/2of PatientsgMG is characterized by debilitating muscleweakness and fatigue. Despite taking an average of2.3 current treatments, 61% of patients have poorwell-being according to WHO-5 Indexhave been diagnosedwith depressionor anxiety in additionto gMGSymptoms can vary from patientto patient, day to day, or eventhroughout the same day.thisunpredictability contributes toemotional burden of diseaseInformation from argenx market research2.6Yearsmean time fromsymptom expression todiagnosis51%of PatientsSurveyed neurologists ranked severe gMG only behindALS as most severe disease they treatstopped workingcompletely fromdisease impact7
VYVGART: First FDA-Approved FcRn AntagonistBlocking FcRnMechanism of Action: VYVGART isa human IgG1 antibody fragmentthat binds to the neonatal Fcreceptor (FcRn), resulting in thereduction of circulating IgG.Reducing IgGRestoring functionIgG AntibodyFcRn, neonatal fragment crystallizable receptor; IgG, immunoglobulin G.(1) Sesarman et al., Cell Mol Life Sci. 2010; (2) Habib et al., Supp Neuro Review. 2020.FcRnVYVGART8
ADAPT ClinicalData ReviewWim Parys, M.D.Chief Medical Officer9
VYVGART Clinical DevelopmentRandomized, double-blindplacebo-controlled Phase 3 trialof 167 adult gMG patientsOngoing single-armopen-label extension studyResults from ADAPTpublished in July 202110
ADAPT Study DesignIndividualized Treatment CyclesDESIGN167 adult gMG patientsMGFA Class II, III, IVAnti-AChR antibody positiveand negativeMG-ADL score 5aOn 1 stable gMG treatmentb2 weeks screeningPatients randomized 1:1 to receive10 mg/kg VYVGART or placeboAll patients receive initialtreatment cycle(1-hour infusion once weekly for 4 weeks)Individualized treatment cycles( 3 cycles in 26 weeks)26 weeksTime between cycles determined by duration ofclinically meaningful improvementcPrimary EndpointOpen-label extension ( 3 years of treatment)MG-ADL responder: 2-point reduction for 4 consecutive weeks,with the first reduction occurring by week 4 during the first cycle (8weeks) in anti-AChR antibody positive patients151 patients who completed ADAPT entered ADAPT studyAChR, acetylcholine receptor; MG-ADL, Myasthenia Gravis Activities of Daily Living; NSIST, nonsteroidal immunosuppressive therapy; MGFA Myasthenia Gravis Foundation of Americaa50% of score attributed to nonocular items; bAcetylcholinesterase inhibitor, steroid, and/or NSIST for the duration of the trial. CCriteria for initiation of next cycle: 8 weeks from start of previous cycle, total MG-ADL 5 points, MG-ADL 2 points of baseline1. Howard JF, et al. Lancet Neurol. 2021;20(7):526-536.11
Treatment Benefit Demonstrated withMG-ADL and QMG Disease ScoresQMG RESPONDERSMG-ADL RESPONDERSP .0001P .000168%63%30%n 44/65VYVGARTn 19/64PlaceboPRIMARYMG-ADL responder: 2-point reduction 4consecutive weeks during thefirst cycle (AChR antibodypositive patients)AChR, acetylcholine receptor; MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, Quantitative Myasthenia GravisHoward JF, et al. Lancet Neurol. 2021;20(7):526-536.14%n 41/65VYVGARTn 9/64PlaceboSECONDARYQMG responder: 3-point reduction for 4consecutive weeks during thefirst cycle (AChR antibodypositive patients)12
Treated Patients Demonstrated Depth of ResponseExploratory EndpointsProportion of AChR-Ab patients with increasingMG-ADL improvement (week 4)Proportion of AChR-Ab patients with MSE (MGADL 0 or 1) during cycle 114.3%40%20.6%27.0%39.7%VYVGARTn acebo7573.0%n 26/658655.6%11%9Minimum Improvementin Total MG-ADL ScoreVYVGART48.3%PlaceboAChR-Ab, acetylcholine receptor antibody; MG-ADL, Myasthenia Gravis Activities of Daily Living; MSE, minimal symptom expressionaHoward JF, et al. Lancet Neurol. 2021;20(7):526-536.13
Safety: Summary of Adverse EventsAEsaSAEsb 1 infusion-related reaction eventcInfection AEsdDiscontinued study treatment due to AEseSevere AEs (grade 3)Most frequent AEso Headacheo Nasopharyngitiso Nauseao Diarrheao Upper respiratory tract infectiono Urinary tract infectionVYVGART (n 84) n (%)Placebo (n 83) n (%)65 (77)4 (5)3 (4)39 (46)3 (4)9 (11)70 (84)7 (8)8 (10)31 (37)3 (4)8 (10)24 (29)10 (12)7 (8)6 (7)9 (11)8 (10)23 (28)15 (18)9 (11)9 (11)4 (5)4 (5)ECG, electrocardiogram; SAE, serious adverse event; aAEs were predominantly mild or moderate in severity. bSAEs efgartigimod group: thrombocytosis, rectal adenocarcinoma, MG worsening (each leading to treatment discontinuation), and depression; SAEs placebo group: 1 case eachof myocardial ischemia, atrial fibrillation, spinal ligament ossification (that led to treatment discontinuation); remaining events were URTI, spinal compression fracture, MG worsening, and MG crisis. cAll infusion AEs were mild in severity. dAll infections were mild to moderate in severityexcept for 3 severe events: influenza and pharyngitis (efgartigimod) and URTI (placebo). eEfgartigimod group: MG worsening, rectal adenocarcinoma, thrombocytosis (all SAEs); placebo group: myocardial ischemia, atrial fibrillation, spinal ligament ossification (all SAEs). Howard JF, et al.Lancet Neurol. 2021;20(7):526-536.14
CommercialStrategyKeith WoodsChief Operating Officer15
Challenge the gMG Treatment ParadigmMeeting our stakeholders where they areEmpower Patientsto Demand BetterBest-in-ClassPatientSupportRapid HCPAdoption ofVYVGARTEnableAppropriateAccess16
17,000 Adult gMG Patients in U.S. Not WellManaged with Current Treatment OptionsAdult MG patients1,265,000Patients progressing to gMG (85% of adult MGpopulation )155,000Patients intolerant to or with inadequate response tocommonly prescribed therapies (37%)120,000Anti-acetylcholine receptor (AChR) antibodypositive patients (85%) 17,000* Patients intolerant to or with inadequate response to prior treatment with conventional therapies based on claims analysis that found that 36.7% of MG patients had progressed past treatment with an acetylcholine esterase inhibitors and/or steroids.1. Gilhus N, et al. Nat Rev Neurol. 2016;12(5):259-268. 2. Meriggioli M, et al. Lancet Neurol. 2009;8(5):475-490. 3. argenx. Data on File from IQVIA PharMetrics Database.17
Infrastructure to Bring VYVGART toPhysicians and Patients*7.7K Neurologists treat 97% of gMG patientsVYVGART Field Teams 71 Territory BusinessManagers 10 Thought Leader Liaisons 16 Medical ResearchLiaisonsInfusion Sitesof CareHome InfusionNursesInformation from argenx market research 10 Nurse Case Managers 13 Market AccessProfessionals 10 Field Reimbursement ManagersNational SpecialtyPharmaciesAccess in all 50States18
Increasing Physician AwarenessAwareness Peer-to-peer speakerprograms Multi-channel HCPactivation Integrated HCP inputinto activation planInterestHCPsEvaluationInitial UseBuildexperiencecurve to driveHCPs fromawareness toadopting use ofVYVGARTwhereappropriateAdoption19
My VYVGART Path isAvailable to Provide AccessSupport and EducationEducate ranceProcess20
Activating the PatientDisease AwarenessDirect-to-Consumer Campaign21
Key Pillars of VYVGART Value-Based AgreementAffordabilityPredictabilitySimplicityThe value-based agreement helps provide cost predictability to participating plansaligning interests between patients, physicians and payers22
Pricing Based on Value to PatientsADAPT Clinical DataSignificantResponseRates(MG-ADL andQMG scales)DemonstratedSafetyReal World Evidence Data gMG imposes significant burden on patients andHCPsIndividualizedDosingExpected annual net pricefor typical VYVGART patient is 225,000 70% of gMG patients not well managed oncurrent treatments Price to vary based on individualized dosing and specificinsurance coverage, and mandatory government rebates anddiscountsHoward et al., 2021, The Lancet Neurology; 20(7):526-536.Howard et al., 2021, AANEM poster - Minimal Symptom Expression in Patients With Generalized Myasthenia Gravis From Treatment With Efgartigimod23
Global Launch Preparations UnderwayGlobalJapanJ-MAA for IV efgartigimod for treatment ofgMG accepted for review by PMDAAnticipated decision in 1Q 2022EUMAA filed with EMA and validatedAnticipated decision in 2H 2022United StatesVYVGART Approved byFDA onDecember 17, 2021ChinaZai Lab to discuss potential acceleratedregulatory pathway for approval in China withNMPAAnticipated filing by mid-2022IsraelPartnership agreement with MedisonAnticipated filing in 2Q 2022Pre-Approval Access Program Openin Europe and Canada24
First Approval In Neuromuscular FranchiseTHIS IS JUST THE BEGINNINGUp to 15indicationsby muneThrombocytopeniaPemphigusChronic lousPemphigoidKIDNEY25
TogetherWe DiscoverReaching Patients ThroughImmunology Innovation26
This presentation does not purport to be all-inclusive or to contain all of the information you may desire. This . thrombocytosis (all SAEs); placebo group: myocardial ischemia, atrial fibrillation, spinal ligament ossification (all SAEs). Howard JF, et al. Lancet Neurol. 2021;20(7):526-536. 14. Commercial Strategy Keith Woods Chief Operating .