Transcription
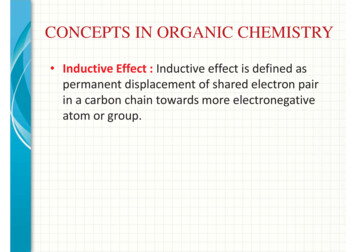
CONCEPTS IN ORGANIC CHEMISTRY Inductive Effect : Inductive effect is defined aspermanent displacement of shared electron pairin a carbon chain towards more electronegativeatom or group.
Types of Inductive effect :1.Negative Inductive Effect : (—I effect,Electron withdrawing effect)when an electronegative atom or group(more electro negative than hydrogen)isattached to the terminal of the carbonchainin a compound, the electronsare displaced in the direction of theattached atom or group.-NO2 -CN -COOH F Cl Br I OH C6H5 H
2. Positive Inductive effect : ( I effect, Electronreleasing effect)When an electro positive atom or group(more electro positive than hydrogen)isattached to the terminal of the carbonchain in a compound, the electrons aredisplaced away from the attached atomor group.(CH3)3C- (CH3)2CH- -C2H5 - CH3.
Applications of Inductive effect:Inductive effect is useful in explaining the strength ofsome organic acids and bases.a) Effect of substituent on the acid strength of aliphaticacids.HCOOH CH3COOH (CH3)2CHCOOHReason : Acidic strength decreases as I effect of the alkylgroup increases.b) O2NCH2COOH FCH2COOH CICH2COOH BrCH2COOH ICH2COOH CH3COOHReason : Acidic strength decreases as -I effect of thegroup or halogen decreases.
Applications of Inductive effect:c) Cl3CCOOH Cl2CHCOOH ClCH2COOH CH3COOHReason : Acidic strength decreases as the number ofhalogen atoms decreases.d) CH3CHClCOOH CH2ClCH2COOHReason : Acidic strength decreases as the distance ofthe halogen from carboxylic group increases.e)i.e., Benzoic acid is stronger than acetic acid.Reason : due to –I effect of phenyl group.
Relative basic strength of amines1. All aliphatic amines are more basic than ammonia.e.g. Methyl amine is more basic than ammonia.Reason : Due to I effect of methyl group.2. Aniline is weaker base than Ammonia.Reason : Due to R effect and –I Effect of phenylgroup.
Electromeric EffectElectromeric effect is defined as the completetransfer of electrons of a multiple bond towardsone of the bonded atoms at the demand of anattacking reagent.Note : a) It is shown by those compoundscontaining multiple bondb) It is a purely temporary effect & isbrought into play only at therequirement of attacking agent.
Types of Electromeric Effect E effect : When displacement of electrons isaway from the atom or group.e.g : addition of H to alkene.-E effect : When displacement of electrons istowards the atom or group.e.g : addition of cyanide ion(CN-) to the carbonylgroup.
Mesomeric/ Resonance EffectThe flow of electrons from one part of aconjugated S system to the other caused byphenomenon of resonance is called resonanceeffect or mesomeric effect.-M or -R effect : When the electron displacementis towards the group.e.g :-NO2 , -CHO, M or R effect : When the electrondisplacement is away from the group.e.g :-OH , -OR,-Cl
SYNTHETIC ORGANIC CHEMISTRY
SYNTHETIC ORGANIC CHEMISTRY
SYNTHETIC ORGANIC CHEMISTRY
ISOMERISM-2Stereo isomerism : Compounds which havethe same molecular formulae and samestructural formulae but differ in spatialarrangement of the atoms or groups areknown as stereoisomers and phenomenon isstereoisomerism.1. Geometrical Isomerism2. Optical Isomerism
1. Geometrical Isomerism : is due torestricted or hindered rotation around thedouble bond.Conditions :1. Molecule must have carbon-carbon double bond .2. There must be two different atoms or groupsattached to each carbon atom of the double bond.
2. Optical Isomerism : Compounds have samemolecular & structural formulae but differ inaction towards plane polarised light.e.g., Lactic acid(2-hydroxypropionic acid)Optical activity : is the ability of chiral substanceto rotate the plane of plane polarized light.Dextrorotatory Compounds : Those compoundsturn the plane of polarized light towards right ( ).Laevorotatory Compounds : Those compoundsturn the plane of polarized light towards left(-).
Cause of Optical activity and Optical Isomerism :Chirality : molecule and its mirror image must benon-superimposable.Chiral Carbon atom : four different groupsattached to carbon atom.Enantiomers : The pair of molecules which arenon-superimposable mirror images of each other.Racemic mixture : when equal amounts of twoenantiomers are mixed together it gives anoptically inactive form.
HYDROCARBONS-2Cycloalkanes: are saturated hydrocarbons inwhich the carbon atoms are joined by singlecovalent bonds to form a ring.General formula CnH2nStability of cycloalkanes :Stability of cycloalkanes increases with thesize of ring upto cyclopentane and fromcyclohexane onwards all cycloalkanes arequite stable.This is explained by Adolf von Baeyer.
Postulates of Baeyer’s Strain Theory:1. The atoms present in any cycloalkanemolecule lie in same plane and thus allcycloalkanes are Co-planar. Carbon-Carbonangle is 1090281.2. The deviation of bond angle from the normaltetrahedral angle is called as anglestrain(1090281).3. The greater the angle strain, the greater is theinstability of ring.4. The higher the stability of the ring thegreater would be its ease of formation.
Calculation of Angle strainAngle strain in various cycloalkane rings
Sachse Mohr Theory of strainless RingsRings with six or more carbon atoms in cycloalkanesare NOT planar but puckered.They exist in two strain less forms boat form andChair formNote : the chair conformation of cyclohexane hasbeen found more stable than boat conformation
Mechanism of Electrophilic SubstitutionReactions of BenzeneBenzene undergoes Electrophilic SubstitutionReactionsReason : due to the presence of S electron cloudabove and below the plane of carbonatoms of the ring.Electrophilic Substitution Reactions are of thefollowing types :Halogenation,Nitration,Sulphonation& Friedel-Craft reaction
HalogenationThe substitution of ‘H’ atom of benzene ring byhalogen atom.Conditions : presence of halogen carrier.(FeCl3or Fe)Electrophile : X (halonium ion)where X Halogen atom
NitrationThe substitution of ‘H’ atom of benzene ring by–NO2 group.To carry out nitration, nitrating mixture is used.Nitrating Mixture : it is a mixture ofConc.HNO3 Conc. H2SO4Electrophile : NO2 (nitronium ion)
SulphonationThe substitution of ‘H’ atom of benzene ring by–SO3H group.To carry out Sulphonation, Conc. sulphuric acidis used.Electrophile : SO3 (Neutral electrophile)
Friedel-Craft ReactionFriedel-Craft alkylationThe substitution of ‘H’ atom of benzene ring by‘alkyl’group.To carry out alkylation, alkyl halide is used.Condition : ‘anhydrous AlCl3’ as catalyst(Lewisacid)Electrophile : CH3 (Methyl Carbocation)
General Mechanism of AromaticElectrophilic Substitution
Elucidation of structure of Benzene1. Molecular Formula : C6H6.2. Possibilty of Open Chain Structure: it ishighly unsaturated, it contains 8 hydrogenatoms less than correspondingalkane(C6H14). Formation of Cyclohexane,benzene hexachloride, benzene triozonideshows that it contains 3 carbon-carbondouble bonds.
Elucidation of structure of Benzene3. Failure of Open Chain Structure :benzene does not decolourise colour ofalk. KMNO4, colour of Br2 water.It undergoes substitution reactionreadily compared to addition reactions,It forms only one type of monosubstitutedproduct.4. Kekule’s Ring Structure:
Elucidation of structure of Benzene5. Objections against Kekule’s structure:Objection-1 : there should exist two orthoisomers for similar substituentsOvercome this objection; kekule suggest thatbenzene is a mixture of two forms in rapidequilibrium.
Elucidation of structure of Benzene5. Objections against Kekule’s structure:Objection-2 : Structure of benzenepossesses three double bonds which suggeststhat it should be highly reactive. But this isagainst the observed fact. Benzene is found tobe stable unlike alkenes(i.e., does not undergothe addition reaction easily).
Elucidation of structure of BenzeneModern view : Structure of benzene can beexplained by Valence bond theory &Molecular Orbital Theory.Valence bond theory :
Elucidation of structure of BenzeneMolecular Orbital Theory.
Types of Inductive effect : 1.Negative Inductive Effect : (—I effect, Electron withdrawing effect) when an electronegative atom or group (more electro negative than hydrogen)is attached to the terminal of the carbon chain in a compound, the electrons are