Transcription
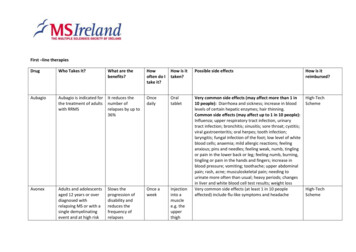
First –line therapiesDrugWho Takes It?What are thebenefits?Howoften do Itake it?How is ittaken?Possible side effectsHow is itreimbursed?AubagioAubagio is indicated forthe treatment of adultswith RRMSIt reduces thenumber ofrelapses by up s and adolescentsaged 12 years or overdiagnosed withrelapsing MS or with asingle demyelinatingevent and at high riskSlows theprogression ofdisability andreduces thefrequency ofrelapsesOnce aweekInjectioninto amusclee.g. theupperthighVery common side effects (may affect more than 1 in10 people): Diarrhoea and sickness; increase in bloodlevels of certain hepatic enzymes; hair thinning.Common side effects (may affect up to 1 in 10 people):Influenza; upper respiratory tract infection, urinarytract infection; bronchitis; sinusitis; sore throat; cystitis;viral gastroenteritis; oral herpes; tooth infection;laryngitis; fungal infection of the foot; low level of whiteblood cells; anaemia; mild allergic reactions; feelinganxious; pins and needles; feeling weak, numb, tinglingor pain in the lower back or leg; feeling numb, burning,tingling or pain in the hands and fingers; increase inblood pressure; vomiting; toothache; upper abdominalpain; rash, acne; musculoskeletal pain; needing tourinate more often than usual; heavy periods; changesin liver and white blood cell test results; weight lossVery common side effects (at least 1 in 10 peopleaffected) include flu-like symptoms and headacheHigh-TechScheme
Betaferonof developing MSPeople with a singledemyelinating eventwith an activeinflammatory process,if it is severe enough towarrant treatmentwith intravenouscorticosteroids, ifalternative diagnoseshave been excluded,and if they aredetermined to be athigh risk of developingclinically definitemultiple sclerosisDelay in theprogression fromfirst clinical event(CIS) to clinicallydefinite MS.Reduction infrequency andseverity of clinicalrelapses.Prolongation ofthe relapse-freeinterval. Delay ction(underthe skin)At the beginning of the treatment adverse reactions arecommon but in general they subside with furthertreatment. The most frequently observed adversereactions are a flu-like symptom complex and injectionsite reactionsHigh-TechSchemeUsed to reducethe number ofrelapsesDailySubcutaneousinjection(underthe skin)Possible allergic reactions (hypersensitivity): signsinclude rash, swelling of the eyelids, face or lips, suddenshortness of breath, convulsions, faintingHigh-TechSchemePeople with RRMSCopaxone20mg/mlPeople with SPMS whostill mobilisePeople with RRMS andpeople who haveexperienced a welldefined first clinicalepisode and aredetermined to be athigh risk of developingclinically definite MSOther reactions following injection (immediate postinjection reaction): flushing or reddening of the chestor face; shortness of breath; chest pain; pounding andrapid heartbeat. These symptoms normally do notcause problems and disappear within half an hour
Common side effects (may affect more than 1 in 10people): infection, flu; anxiety, depression; headache;feeling sick; skin rash; pain in the joints or back; feelingweak; skin reactions at the injection site includingreddening of skin, pain, formation of wheals, itching,tissue swelling, inflammation and hypersensitivity; nonspecific painCopaxone40mg/mlPeople with RRMSUsed to reducethe number ofMS relapsesThreetimes perweek,with aminimumof rthe skin)Possible allergic reactions (hypersensitivity): signsinclude rash, swelling of the eyelids, face or lips, suddenshortness of breath, convulsions, faintingHigh-TechSchemeOther reactions following injection (immediate postinjection reaction): flushing or reddening of the chestor face; shortness of breath; chest pain; pounding andrapid heartbeat. These symptoms normally do notcause problems and disappear within half an hourCommon side effects (may affect more than 1 in 10people): infection, flu; anxiety, depression; headache;feeling sick; skin rash; pain in the joints or back; feelingweak; skin reactions at the injection site includingreddening of skin, pain, formation of wheals, itching,tissue swelling, inflammation and hypersensitivity; nonspecific painGilenyaPeople whoseneurologists considertheir disease is rapidly54% relapse ratereduction butalso benefits onOncedailyOralThe five main safety areas of interest are: Bradycardia(slowing heart rate) on first dose; macular oedema (aneye problem) for which you will be examined withinHigh-TechScheme
LemtradaPlegridyevolvingdisability, MRIand brain atrophyPeople with RRMS withactive disease definedby clinical or imagingfeaturesReduces thenumber of MSrelapses andhelps to slowdown or reversesome of the signsand symptoms ofMSAdults with RRMSSlows theprogression ofdisability anddecreases thethree to four months of initiating Gilenya; lowlymphocyte count; raised liver enzymes.Administered in twotreatmentcourses.For thefirsttreatmentcoursepeoplereceiveoneinfusionper dayfor fivedays. Oneyear laterpeoplereceiveoneinfusionper dayfor threedays.Onceevery twoweeksInfusionCommon side effects (more than 1 in 100): thyroiddisordersNational DrugsManagementSchemeLess common (less than 1 in 100): idiopathicthrombocytopenic purpura is a disorder that preventsthe blood from clotting. Kidney disease is also a majorbut rare side effect (may affect up to 1 in 1,000 people)Injectionunder theskin ofthe thigh,Very common side effects (at least 1 in 10 peopleaffected) include: flu-like symptoms; headache; musclepain; pain in joints/arms/legs/neck; chills; fever; feelingweak and tired; injection site reactionsHigh-TechScheme
frequency ofrelapsesRebifPeople with RRMSTecfideraAdults with RRMSTysabriAdults with highlyactive rapidly evolvingsevere relapsingremitting MSReduces thenumber andseverity ofrelapses andslows theprogression ofdisability. Alsoapproved for usefor people with adiagnosis of CIS.Slows theprogression ofdisability andreduces thefrequency ofrelapsesDisabling effectsof MSapproximatelyhalved and thenumber of MSattacks decreasedby two-thirds inclinical trialsThreetimes perweekabdomenor upperarmSubcutaneousinjection(underthe skin)Common side effects (more than 1 in 100 peopleaffected) include: flu-like symptoms; injection sitereactions; liver enzyme abnormalities; headache;depression; nausea or vomiting; difficulty sleeping; hairloss.High-TechSchemeLess common side effects (less than 1 in 100 peopleaffected): thyroid dysfunction; allergic reactions; liverinflammation; increased sweating; blood clotsTwice adayOralcapsuleVery common side effects (at least 1 in 10 peopleaffected) include: flushing; diarrhoea; nausea, stomachpain/crampsHigh-TechSchemeOnce amonthInfusioninto aveinVery common side effects (at least 1 in 10 peopleaffected) include infusion reactions e.g. dizziness,feeling sick, itchy rash and shivering.Progressive multifocal leukoencephalopathy (PML) is anuncommon but serious side effect.National DrugsManagementScheme
Second-line therapiesDrugWho takes it?What are thebenefits?GilenyaPeople who havefailed on a first-linetreatmentTysabriAdults with relapsingremitting MS whohave high diseaseactivity despitetreatment with otherDMTsZinbrytaAdults with relapsingremitting MS whohave had an adequateresponse to two ormore other DMTsFewer relapses,reduction in thenumber and sizeof MRI lesions,reduction in brainvolume loss, lesslikely toexperienceworsening ofdisabilityDisabling effectsof MSapproximatelyhalved and thenumber of MSattacks decreasedby two-thirds inclinical trialsReduced annualrelapse rate by45% compared tobeta interferonHowoften do Itake it?OncedailyHow is ittaken?Possible side effectsHow is itreimbursed?OralThe five main safety areas of interest are:Bradycardia (slowing heart rate) on first dose;macular oedema (an eye problem) for which you willbe examined within three to four months ofinitiating Gilenya; low lymphocyte count; raised liverenzymes.High-TechSchemeOnce amonthInfusioninto aveinVery common side effects (at least 1 in 10 peopleaffected) include infusion reactions e.g. dizziness,feeling sick, itchy rash and shivering.Progressive multifocal leukoencephalopathy (PML) isan uncommon but serious side effect.NationalDrugsManagementSchemeOnce amonthSubcutaneousinjectionReported side effects included serious infections,serious skin reactions, headaches, cold and flu-likesymptoms and fever. Impaired liver function wasalso reported and it is recommended that liverfunction testing should be conducted prior tocommencing treatment with Zinbryta, and should becontinually monitored while on this treatment.High-TechScheme
Symptomatic treatmentsDrugWho takes it?FampyraAdults with MSrelated walkingdisabilityWhat are thebenefits?Walking speedimprovementKey: RRMS Relapsing remitting multiple sclerosisCIS Clinically Isolated SyndromeSPMS Secondary progressive multiple sclerosisHow often do Itake it?Twice dailyHow is it taken?Oral tabletPossible side effectsVery common sideeffects includeurinary tractinfectionHow is itreimbursed?Reimbursed by theHSE on a responderbasis
injection (under the skin) At the beginning of the treatment adverse reactions are common but in general they subside with further treatment. The most frequently observed adverse reactions are a flu-like symptom complex and injection site reactions High-Tech Scheme Copaxone 20mg/ml People with RRMS and people who have experienced a well-