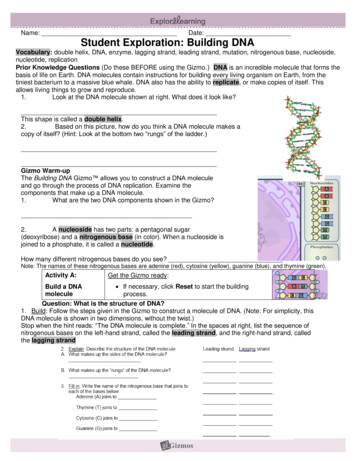
Transcription
Topics in ForensicDNA Analysis &InterpretationIndiana DNATraining WorkshopIndianapolis, INMarch 28, 2011Dr. John M. ButlerNational Institute ofStandards and Technologyjohn.butler@nist.gov
Background on the PresenterJohn M. Butler has a B.S. in chemistry from Brigham Young University and a Ph.D. inanalytical chemistry from the University of Virginia. His dissertation research, which wasconducted at the FBI Academy in Quantico, VA, involved pioneering work in applying capillaryelectrophoresis to STR typing. While a postdoc at NIST, he designed and built STRBase, thewidely used Short Tandem Repeat Internet Database (http://www.cstl.nist.gov/biotech/strbase)that contains a wealth of standardized information on STRs used in human identityapplications. He worked for several years as a staff scientist and project leader at a Californiastartup company named GeneTrace System developing rapid DNA analysis technologiesinvolving time-of-flight mass spectrometry. In the fall of 1999, he returned to NIST to lead theirefforts in human identity testing with funding from the National Institute of Justice.Dr. Butler is currently a NIST Fellow and Group Leader of Applied Genetics in the BiochemicalScience Division at the National Institute of Standards and Technology. He is a regular invitedguest of the FBI’s Scientific Working Group on DNA Analysis Methods (SWGDAM) and amember of the Department of Defense Quality Assurance Oversight Committee for DNAAnalysis. Following the terrorist attacks of 11 September 2001, he aided the DNA identificationefforts and served as part of the distinguished World Trade Center Kinship and Data AnalysisPanel (WTC KADAP). He is a member of the International Society of Forensic Genetics andserve as an Associate Editor for Forensic Science International: Genetics.Dr. Butler has received numerous awards including the Presidential Early Career Award forScientists and Engineers (2002), the Department of Commerce Silver Medal (2002) and GoldMedal (2008), the Arthur S. Flemming Award (2007), the Edward Uhler Condon Award (2010),Brigham Young University’s College of Physical and Mathematical Sciences Honored Alumnus(2005), and the Scientific Prize of the International Society of Forensic Genetics (2003).He has more than 100 publications describing aspects of forensic DNA testing and is one ofthe most prolific active authors in the field with articles appearing regularly in every majorforensic science journal. Dr. Butler has been an invited speaker to numerous national andinternational forensic DNA meetings and in the past few years has spoken in Germany,France, England, Canada, Mexico, Denmark, Belgium, Poland, Portugal, Cyprus, TheNetherlands, Argentina, Japan, and Australia. In addition to his busy scientific career, he andhis wife serve in their community and church and are the proud parents of six children, all ofwhom have been proven to be theirs through the power of DNA typing.For listing of publications, see m.
Topics in Forensic DNA Analysis & InterpretationDNA Workshop for Indiana State Police LaboratoryInstructor: John M. Butler (NIST)Indianapolis, INMarch 28, 2011Proposed Agenda (to start at 8:00 a.m.)Introductions (15 minutes)CE Fundamentals & Troubleshooting (60 minutes)ABI 3500 (15 minutes)- BREAK (15 minutes) -Low-level DNA Issues (30 minutes)Validation Discussion (60 minutes) – discuss specific on-going validation studiesThoughts on the Future Directions of the Field (15 minutes)- LUNCH (60 minutes) -Mixtures & SWGDAM Interpretation Guidelines (120 minutes)- BREAK (15 minutes) Y-STRs (30 minutes)Relationship Testing & Parentage Statistics (30 minutes)Additional Q & A (15 minutes)Please ask questions throughout the presentations!
Recommended LiteratureCompiled by John Butler (March 2011)CE Fundamentals and TroubleshootingButler, J.M., et al. (2004) Forensic DNA typing by capillary electrophoresis using the ABI Prism 310 and 3100 genetic analyzers for STR analysis.Electrophoresis 25: 1397-1412.Lazaruk, K., et al. (1998) Genotyping of forensic short tandem repeat (STR) systems based on sizing precision in a capillary electrophoresisinstrument. Electrophoresis 19: 86-93.McCord, B.R. (2003) Troubleshooting capillary electrophoresis systems. Profiles in DNA 6(2): 10-12. Available at: http://www.promega.com/profiles/.Moretti, T.R., et al. (2001) Validation of short tandem repeats (STRs) for forensic usage: performance testing of fluorescent multiplex STR systemsand analysis of authentic and simulated forensic samples. J. Forensic Sci. 46: 647-660.Low-level DNA DNA.htm)Benschop, C.C.G., et al. (2010) Low template STR typing: effect of replicate number and consensus method on genotyping reliability and DNAdatabase search results. Forensic Sci. Int. Genet. (in press). doi:10.1016/j.fsigen.2010.06.006Butler, J.M., & Hill, C.R. (2010) Scientific issues with analysis of low amounts of DNA. Profiles in DNA 13(1). Available athttp://www.promega.com/profiles/1301/1301 02.html.Whitaker, J. P., et al. (2001) A comparison of the characteristics of profiles produced with the AMPFlSTR SGM Plus multiplex system for bothstandard and low copy number (LCN) STR DNA analysis. Forensic Sci. Int. 123: h/strbase/validation.htm)Butler, J.M. (2006) Debunking some urban legends surrounding validation within the forensic DNA community. Profiles in DNA 9(2): 3-6. Available athttp://www.promega.com/profiles/.ENFSI DNA Working Group (2010) Recommended minimum criteria for the validation of various aspects of the DNA profiling process. Available athttp://www.enfsi.eu.SWGDAM. (2004) Revised validation guidelines. Forensic Science Communications, 6(3). Available /standards/2004 03 standards02.htmFuture Directions of the FieldButler, J.M., et al. (2007) STRs vs SNPs: thoughts on the future of forensic DNA testing. Forensic Sci. Med. Pathol. 3: 200-205.Kayser, M., & de Knijff, P. (2011) Improving human forensics through advances in genetics, genomics and molecular biology. Nature Rev. Genet. 12:179-192.Mixtures and SWGDAM Interpretation Guidelines htm)Clayton, T.M., et al. (1998) Analysis and interpretation of mixed forensic stains using DNA STR profiling. Forensic Sci. Int. 91: 55-70.Gill, P., et al. (2006) DNA commission of the International Society of Forensic Genetics: Recommendations on the interpretation of mixtures. ForensicSci. Int. 160: 90-101.SWGDAM. (2010) SWGDAM interpretation guidelines for autosomal STR typing by forensic DNA testing laboratories. Available .gov/biotech/strbase/y str.htm)Brenner, C.H. (2010) Fundamental problem of forensic mathematics—the evidential value of a rare haplotype. Forensic Sci. Int. Genet. 4: 281-291.Buckleton, J.S., et al. (2011) The interpretation of lineage markers in forensic DNA testing. Forensic Sci. Int. Genet. 5: 78-83.Gusmão, L. et al. (2006) DNA Commission of the International Society of Forensic Genetics (ISFG): an update of the recommendations on the use ofY-STRs in forensic analysis. Forensic Sci. Int. 157: 187-197.Krenke, B.E., et al. (2005) Validation of male-specific, 12-locus fluorescent short tandem repeat (STR) multiplex. Forensic Sci. Int. 151: 111-124.Relationship inship.htm)Gjertson, D.W. et al. (2007) ISFG: recommendations on biostatistics in paternity testing. Forensic Sci. Int. Genet. 1(3-4): 223-231.
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011Topics in Forensic DNA Analysis & InterpretationDr. John M. ButlerNIST Fellow & Applied Genetics Group ler.htmExperienceIntroductionsIndiana DNATraining WorkshopDr. John M. ButlerNational Institute ofStandards and TechnologyIndianapolis, INjohn.butler@nist.govMarch 28, 2011 University of Virginia/FBI Laboratory (1992-1995)– Work performed in Bruce McCord’s labContact Informationjohn.butler@nist.gov NIST NRC Postdoc (1995-1997)301-975-4049 GeneTrace Systems Inc (1997-1999) NIST Human Identity Project Leader (1999-present) Ph.D. dissertation (Aug 1995): “Sizing and quantitationof polymerase chain reaction products by capillaryelectrophoresis for use in DNA typing” Forensic DNA Typing textbook (now in its 3rd Edition) STRBase website: http://www.cstl.nist.gov/biotech/strbase/ Family: wife Terilynne and 6 children Hobbies: reading, writing, and making PowerPoint slidesLocation of NISTNIST History and MissionBaltimore, MD National Institute of Standards and Technology(NIST) was created in 1901 as the NationalBureau of Standards (NBS). The name waschanged to NIST in 1988.I-270I-95NISTAFDIL NIST is part of the U.S. Department ofCommerce with a mission to develop andpromote measurement, standards, andtechnology to enhance productivity, facilitatetrade, and improve the quality of life.BWIAirportCapitol Beltway(I-495)WashingtonD.C. 686 for 3 jarsReaganNationalAirportDullesAirport NIST supplies over 1,300 Standard ReferenceMaterials (SRMs) for industry, academia, andgovernment use in calibration ofmeasurements.I-66I-95FBI NIST defines time for the U.S.LabDNA typing standardRichmond, VANIST Human Identity Project Teamswithin the Applied Genetics GroupForensic DNA TeamJohnButlerMikeCobleBeckyHillData AnalysisSupportMargaretKlineFunding from the National Institute of Justice (NIJ)through NIST Office of Law Enforcement StandardsDaveDuewerCurrent Areas of NIST Effort with Forensic DNADNA Biometrics Team Standards– Standard Reference Materials– Standard Information Resources (STRBase website)– Interlaboratory StudiesPeteValloneEricaButtsKristen LewisO’ConnorFunding from the FBI S&T Branchthrough NIST Information Access Division Technology– Research programs in SNPs, miniSTRs, Y-STRs,mtDNA, qPCR– Assay and software development, expert system review Training Materials– Review articles and workshops on STRs, CE, validation– PowerPoint and pdf files available for base/training.htm1
J.M. Butler – Indiana DNA Training WorkshopContributors to These Workshop SlidesMarch 28, 2011Forensic Science International: :Angel Carracedo (Spain)Associate Editors:Peter M. Schneider (Germany)John M. Butler ISTNISTCEABI3500low levelDNADNAmixtureskinshipanalysisPrimary Sources for MaterialCovered in this WorkshopFSI: Genetics is a new journaldedicated exclusively to thefield of forensic genetics. It hasWe need your helpas good reviewersand authorsbeen launched in 2007 by ElsevierPublishers in affiliation with theInternational Society of ForensicGenetics. All members of the ISFGreceive a free subscription ofthis journal (print and online version)as part of their membership benefits.3rd Edition is Three Volumes See recommended reference listAdvanced Topics in Butler, J.M. (2009) Fundamentals of Forensic DNA Typing.Elsevier Academic PressForensicDNA Typing:INTERPRETATION Butler, J.M. (2011) Advanced Topics in Forensic DNA Typing:Methodology. Elsevier Academic PressSept 2009 NIST STRBase website: http://www.cstl.nist.gov/biotech/strbase/Sept 2011Fall 2012These workshop materials are available g.htmParticipants’ BackgroundsNIST and NIJ DisclaimerFunding: Interagency Agreement 2008-IJ-R-029between the National Institute of Justice and NISTOffice of Law Enforcement StandardsPoints of view are mine and do not necessarily representYour nameYour organization/labExperience level (e.g., 1 yr, 5 yr, 20 yr)What you hope to learn todaythe official position or policies of the US Department of Justice or theNational Institute of Standards and Technology.Certain commercial equipment, instruments and materials are identifiedin order to specify experimental procedures as completely aspossible. In no case does such identification imply arecommendation or endorsement by the National Institute ofStandards and Technology nor does it imply that any of thematerials, instruments or equipment identified are necessarily thebest available for the purpose.Our publications and presentations are made available ning.htm2
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011Topics in Forensic DNA Analysis & InterpretationPresentation OutlineCapillary Electrophoresis:Fundamentals & Troubleshooting NIST experience with DNA analysis using CE CE separation, injection, detection process Troubleshooting tips and suggestionsIndiana DNATraining WorkshopDr. John M. ButlerNational Institute ofStandards and TechnologyIndianapolis, INjohn.butler@nist.govMarch 28, 2011J.M. Butler (2011) Advanced Topics in Forensic DNA Typing: Methodology, Table 6.1Genetic Analyzers from Applied BiosystemsABI GeneticAnalyzer373(gel system)377(gel system)Years Releasedfor Human IDNumber ofCapillariesLaserPolymerdelivery1992-2003--40 mW Ar (488/514 nm)--PMTs and color filter wheelfor detection1995-2006--40 mW Ar (488/514 nm)--CCD cameraOther featuresABI Genetic Analyzer Usage at NIST(All instruments were purchased using NIJ funds)ABI 310Single capillary 1st was purchased in 1996 as Mac (A230, now B233) 2nd was purchased in June 2002 as NT (B261)3101995-110 mW Ar (488/514 nm)syringe31002000-20051625 mW Ar (488/514 nm)syringeABI 3100 Æ 3130xl3100-Avant2002-2007425 mW Ar (488/514 nm)syringe 1st purchased in April 2001 as ABI 310031302003-2011425 mW Ar (488/514 nm)pump3130xl2003-20111625 mW Ar (488/514 nm)pump35002010-810-25 mW diode(505 nm)new pumpMac operating system &Windows NT (later)– upgraded to 3130xl in Sept 2005– Located in a different room (A230, now B219) 2nd purchased in June 2002 as ABI 3100110V power; RFID-taggedreagents; .hid files;normalization & 6-dyedetection possible– Original data collection (v1.0.1) software retained– updated to 3130xl in Jan 2007 (B219, now B261)3500xl2010-2437002002-20039625 mW Ar (488/514 nm)cuvettebased37302005-4825 mW Ar (488/514 nm)pumpABI 35009625 mW Ar (488/514 nm)pump Purchased Nov 2010 (B233)3730xl2005-16 capillariesSplit beam technology8 capillariesInformation courtesy of Michelle S. Shepherd, Applied Biosystems, LIFE Technologies.DNA Samples Run at NISTwe have processed 100,000 samples (from 1996-present) STR kits– Identifiler, PP16, PP16HS, Identifiler Plus, Identifiler Direct,Profiler Plus, Cofiler, SGM Plus, ESI/ESX 17, SE33 monoplexCapillary Electrophoresis Argon IonLaser50-10050-100 µmµm xx 2727 cmcm- Research & development on new assays– STRs: Y-STR 20plex, MeowPlex, miniSTRs, 26plex– SNPs: SNaPshot assays: mtDNA (one 10plex), Y-SNPs (four6plexes), Orchid SNPs (twelve 6plexes), ancestry SNPs (two12plexes), SNPforID (one 29plex), SNPplex (one inutes.minutes.Inlet(cathode) DNA sequencing– Variant allele sequencingSample trayWe have a unique breadth and depth of experience with these instruments htm Burn capillarywindowSample tray movesautomatically beneath thecathode end of the capillary todeliver each sample sitionAcquisitionandandAnalysisAnalysis1
J.M. Butler – Indiana DNA Training WorkshopReview Article on STRs and CEpdf available from tmMarch 28, 2011Analytical Requirements for STR TypingButler et al. (2004) Electrophoresis 25: 1397-1412Raw data (w/ color overlap) Fluorescent dyes must bespectrally resolved in orderto distinguish different dyelabels on PCR productsSpectrally resolved PCR products must bespatially resolved – desirableto have single base resolutionout to 350 bp in order todistinguish variant alleles High run-to-run precision –an internal sizing standard isused to calibrate each run inorder to compare data overtimeArgon ionLASER(488 nm)SizeSeparationSampleSeparationSteps in STR Typingwith ABI 310/3100Detection with Multiple Capillaries(Irradiation for Capillary Arrays)ABI xcitation(488 nm)Capillary(filled withpolymersolution)CCD Panel (with virtual filters)LASERSample DetectionExcitation(488 nm)SampleInjectionLASERProcessing with GeneScan/Genotyper softwareExcitationMixture of dye-labeledPCR products frommultiplex PCR reaction(488 nm)Capillary ArraySamplePreparationSample InterpretationButler, J.M. (2005) Forensic DNA Typing, 2nd Edition, Figure 13.8, Elsevier Science/Academic PressSide irradiation(on-capillary)ABI 3100, 3130, 3100AvantSheath flow detectionFixed laser,moving capillariesABI 3700MegaBACEProcess Involved in 310/3100 Analysis Separation––––Capillary – 50um fused silica, 43 cm length (36 cm to detector)POP-4 polymer – Polydimethyl acrylamideBuffer - TAPS pH 8.0Denaturants – urea, pyrolidinoneSeparation Injection– electrokinetic injection process (formamide, water)– importance of sample stacking Detection– fluorescent dyes with excitation and emission traits– CCD with defined virtual filters produced by assigning ase/training.htm2
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011Separation IssuesOhm’s Law Electrophoresis buffer ––––– V IR (where V is voltage, I is current, and R is resistance) Current, or the flow of ions, is what matters most inelectrophoresisUrea for denaturing and viscosityBuffer for consistent pHPyrolidinone for denaturing DNAEDTA for stability and chelating metals Polymer solution -- POP-4 (but others work also) CE currents are much lower than gels because of ahigher resistance in the narrow capillary Capillary wall coating -- dynamic coating with polymer CE can run a higher voltage because the capillaryoffers a higher surface area-to-volume ratio and canthus dissipate heat better from the ion flow (current) Run temperature -- 60 oC helps reduce secondarystructure on DNA and improves precision.(Temperature control affects DNA sizing)– Wall charges are masked by methyl acrylamideCapillary Wall Coatings Impact DNA SeparationsWhat is in POP-4 and Genetic Analyzer Buffer?Electrophoretic flow -See also Wenz et al. (1998) Genome Research 8: 69-80POP-4 (4% poly-dimethylacrylamide, 8 M urea, 5% 2-pyrrolidinone)US Patent 5,552,028 covers POP-4 synthesisNRunning buffer contains 100 mMTAPS and 1 mM EDTA (adjustedto pH 8.0 with NaOH) TAPS NTris-(hydroxymethyl)methyl-3aminopropane-sulfonic acidON DNA-DNA-- Capillary WallSiOHSiO- H OOOONNNODNA-EOF Bulk FlowElectroosmotic flow (EOF)Solvated ions drag solution towards cathode in a flat flow profileN(a)How to Improve Resolution?1. Lower Field StrengthGelLarger DNA molecules interactmore frequently with the gel and arethus retarded in their migrationthrough the gel2. Increase Capillary Length(b)GelLong DNAmolecules3. Increase Polymer ConcentrationSmall DNAmolecules4. Increase Polymer LengthOgston trbase/training.htmAll of these come at a cost of longer separation run times3
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011Impact of Capillary Length and Polymer Concentrationon DNA Sequencing Resolution310 POP4-20min (36cm)310 POP4-30min (36cm)Injection310 POP6-36min (36cm)310 POP6-50min (36cm)310 POP6-120min (36cm)310 POP6-120min (50cm)3130 POP7-120min (80cm)Longer run timesat lower voltageBigDye Terminator v3.1 Sequencing Kit (Sample: pGEM)Data collected at NIST by Tomohiro Takamaya (Japanese guest researcher, fall 2007)Electrokinetic Injection ProcessCapillary and Electrode Configurations(a) nt of DNA injected isinversely proportional to theionic strength of the solutionSalty samples result inpoor injectionsDNA-DNA----PCR productsin formamideor waterDNA-DNA-SampleTubeMulti-CapillaryElectrode ConfigurationSample TubeABI 310Sample Conductivity Impacts Amount InjectedElectrode adjacent to Ainj] Et(πr2) (μep μeof)[DNAsample] (λbuffer)[DNAinj] is the amount of sample injectedλsample[DNAsample] is the concentration ofDNA in the sampleE is the electric field appliedt is the injection timer is the radius of the capillaryλbuffer is the buffer conductivityλsample is the sample conductivityμep is the mobility of the sample moleculesμeof is the electroosmotic mobilityABI 3100Individual electrode surrounds each training.htmButler et al. (2004) Electrophoresis 25: 1397-1412Cl- ions and other buffer ions present inPCR reaction contribute to the sampleconductivity and thus will compete withDNA for injection onto the capillary4
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011Steps Performed in Standard ModuleComments on Sample PreparationSee J.M. Butler (2005) Forensic DNA Typing, 2nd Edition; Chapter 14 Capillary fill – polymer solution is forced into the capillary by applying a force tothe syringePre-electrophoresis – the separation voltage is raised to 10,000 volts and runfor 5 minutes;Water wash of capillary – capillary is dipped several times in deionizedwater to remove buffer salts that would interfere with the injectionprocessSample injection – the autosampler moves to position A1 (or the next samplein the sample set) and is moved up onto the capillary to perform the injection; avoltage is applied to the sample and a few nanoliters of sample are pulled ontothe end of the capillary; the default injection is 15 kV (kilovolts) for 5 secondsWater wash of capillary – capillary is dipped several times in waste water toremove any contaminating solution adhering to the outside of the capillaryWater dip – capillary is dipped in clean water (position 2) several timesElectrophoresis – autosampler moves to inlet buffer vial (position 1) andseparation voltage is applied across the capillary; the injected DNA moleculesbegin separating through the POP-4 polymer solutionDetection – data collection begins; raw data is collected with no spectraldeconvolution of the different dye colors; the matrix is applied during Genescananalysis Use high quality formamide ( 100 μS/cm) Denaturation with heating and snap cooling isnot needed (although most labs still do it ) Post-PCR purification reduces salt levels andleads to more DNA injected onto the capillaryRemoval of Dye Artifacts Following PCR AmplificationNo Filtering (Straight from PONote higherRFU valuesdue to saltreduction withspin columnsFGAEDGE GELFILTRATIONCARTRIDGESD21S11D7S820Butler, J.M., Shen, Y., McCord, B.R. (2003) The development of reduced size STR amplicons as tools for analysis of degradedDNA. J. Forensic Sci 48(5) 1054-1064.Optics for ABI 310FluorescenceCCD DetectorEmissionS’1Focusing MirrorRe-imaging LensLong Pass FilterArgon-IonLaserenergyhνex 1Capillary Holder(488/514 tionGratingStokesshift13Soλex maxLaser ShuttersMicroscope Objective Lensλem maxWavelength (nm)Laser FilterDiverging LensDichroic ining.htm5
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011The polymerase chain reaction (PCR) is used toamplify STR regions and label the amplicons withfluorescent dyes using locus-specific primersMethods for Fluorescently Labeling DNAUnlabeled DNA8 repeats(a)EthidiumbromideSYBR GreenIntercalator insertsbetween base pairs ondouble-stranded DNALocus 110 repeatsDNA labeled withintercalating dye8 repeatsLocus 29 repeatsFluorescent dNTPs are incorporatedinto both strands of PCR product(b)(c)Fluorescent dyelabeled primerOne strand of PCR product islabeled with fluorescent dyeScannedGel ImageABI Fluorescent Dyes Used in Four-Color DetectionFAMJOE(blue)(green)Capillary ElectropherogramFluorescent Emission Spectra for ABI Dyes5-FAM JOE NEDROXNED is a brighterdye than TAMRATAMRAROX(yellow)(red)Normalized FluorescentIntensity100806040200520540560580 600620640WAVELENGTH (nm)Laser excitation(488, 514.5 nm)ABIABI 310310FilterFilterSetSet FFButler, J.M. (2001) Forensic DNA Typing, Figure 10.4, Academic Press(a)Scan numberImportance of Spectral CalibrationRelative Fluorescence UnitsBefore Color SeparationRegion shown belowRelative Fluorescence UnitsAfter Color Separation(b)DNA size in base ning.htmABI 310 Data6
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 20115 x 5 matrix for 5-dye analysis on ABI 310Matrix with 4 Dyes on ABI 310I540 bxb gyb yzb rwbI560 bxg gyg yzg rwgI580 bxy gyy yzy rw yI610 bxr gyr yzr yw rintensity of blueintensity of greenintensity of yellowintensity of redWhereb is the %blue labeled DNAg is the %green labeled DNA, etc.From Identifiler User’s ManualProcessed Data (matrix applied with baselining)Raw Data for Matrix Standards6FAMx,y,z,w are the numbers in thematrix (sensitivity to each color)VICNEDIf you solve xyzw for each dye individuallyThen you can determine dye contribution for any mixtureVirtual Filters Used in ABI 310PETLIZVariable Binning Increases Red PeaksComparison of Data Collection VersionsVisible spectrum range seen in CCD camera525500550575600625650675 700 nm(b)(a)Commonly usedHEXPET ROXLIZfluorescent dyesFLTET JOE NEDTMRFAMVICArrows indicate the dye emission spectrum maximumFilter sets determine whatregions of the CCD cameraare activated and thereforewhat portion of the visiblelight spectrum is collectedFilter AFilter CFilter FABI 3100ABI 3130xlData Collection v1.0.1Data Collection v3.0Filter G5Filter AFilter CFilter FFilter EDNEDRedCXRROXROXPETOrangeLIZUsed with These KitsPowerPlex 16in-house assaysProfiler PlusIdentifilerDeciphering Artifacts from the True AllelesBiological (PCR)artifactsThe same PCR products examined with different data collection versions. In (a) thereis an equal number of pixels of light collected from the CCD camera for the bluelabeled and red-labeled peaks. In (b) the signal increase in the red dye-labeled PCRproducts is accomplished with ‘variable binning’ where more pixels of light arecollected from the CCD camera in the red-channel to help balance the less sensitivered dye with blue dye-labeled amplicons.NIST ABI 3100 Analysis Using POP-6 PolymerSTR allelesHigh ResolutionSTR TypingStutter productsSNaPshot SNP Typing(Coding Region mtSNP 11plex minisequencing assay)spikeDye blob6.0%stutter7.8%Blue channelD3S1358Incompleteadenylation A A-A-AGreen channelPull-up(bleed-through)Yellow channelmtDNA Sequencing (HV1)Red channelD8S1179Butler, J.M. (2005) Forensic DNA Typing, 2nd Edition, Figure 15.4, Elsevier Science/Academic ning.htm7
J.M. Butler – Indiana DNA Training WorkshopMaintenance of ABI 310/3100/3130 Syringe – leaks cause capillary to not fillproperly Capillary storage & wash – it dries, it dies! Pump block – cleaning helps insure good fill Change the running buffer regularlyYOU MUST BE CLEAN AROUND A CE!March 28, 2011Protocols Used for STR Typing Most forensic DNA laboratories follow PCRamplification and CE instrument protocols providedby the manufacturer Comments– Lower volume reactions may work fine and reduce costs– No heat denaturation/snap cooling is required prior toloading samples into ABI 310 or ABI 3100– Capillaries do not have to be thrown away after 100 runs– POP-4 polymer lasts much longer than 5 days on an ABI310– Validation does not have to be an overwhelming taskBruce McCord’sProfiles in DNA ArticleVolume 6 (2), Sept 2003, pp. 10-12TroubleshootingExternal FactorsEffect of temperature on allele sizeFGA Allele 30268 Room temperature265Size– Variations in room temperature can cause mobility shifts withband shifts and loss of calibration– Temperature is also important due to effects of highhumidity on electrical conductance262259256253 Cleanliness– Urea left in sample block can crystallize and catalyze furthercrystal formation causing spikes, clogs and other problems.– Best bet is to keep polymer in system and not remove orchange block until polymer is used up.4045505560657075TemperatureSlope is 0.14 bases/degree centigradeTherefore a small change in temperature has a big effect(A 1-2 degree shift in temperature of the heat plate can produce an OL allele)Hartzell, B., et al. (2003). Response of short tandem repeat systems to temperature and sizing methods.Forensic Science International, 133, raining.htm8
J.M. Butler – Indiana DNA Training WorkshopTemperature EffectsOffOff-Ladder “OL Alleles”Alleles”March 28, 2011“OL alleles ” - look at the 250 peak-0.44 bp“OL allele rere-injected”injected”And the 250 peak.-0.12 g.htm9
J.M. Butler – Indiana DNA Training WorkshopMarch 28, 2011TemperatureProbesMonitoring Room Temperature Over TimeFrig/Freeze Monitors 240#DT-23-33-80 – USB Temperature DataloggerPLUS Software 79.00 (#DT-23-33-60)Refrigerator and freezer monitoringRoom Monitors, # DT-23039-52 – USBTemperature-Humidity Datalogger 91.00( Cole Parmer, Vernon Hills IL)Room temperature monitoringoC 10spread(over many weeks)Monitoring Instrument Room Temperature Fluctuations227/A230TemperatureMonitoring of twoseparateinstrument rooms.Poor Temperature ControlCauses DNA Sizing ImprecisionBox area is a 24hour period where
(2005), and the Scientific Prize of the International Society of Forensic Genetics (2003). He has more than 100 publications describing aspects of forensic DNA testing and is one of the most prolific active au