Transcription
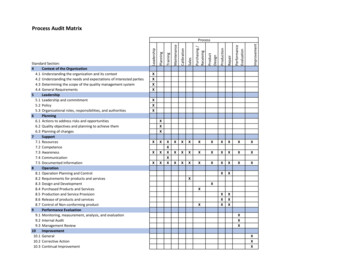
Process Audit MatrixCalibrationSalesPurchasing andard Section:4Context of the Organization4.1 Understanding the organization and its context4.2 Understanding the needs and expectations of interested parties4.3 Determining the scope of the quality management system4.4 General Requirements5Leadership5.1 Leadership and commitment5.2 Policy5.3 Organizational roles, responsibilities, and authorities6Planning6.1 Actions to address risks and opportunities6.2 Quality objectives and planning to achieve them6.3 Planning of changes7Support7.1 Resources7.2 Competence7.3 Awareness7.4 Communication7.5 Documented information8Operation8.1 Operation Planning and Control8.2 Requirements for products and services8.3 Design and Development8.4 Purchased Products and Services8.5 Production and Service Provision8.6 Release of products and services8.7 Control of Non-conforming product9Performance Evaluation9.1 Monitoring, measurement, analysis, and evaluation9.2 Internal Audit9.3 Management Review10Improvement10.1 General10.2 Corrective Action10.3 Continual XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
Process NameDocuments Defining the ProcessProcess InputsProcess OutputsLeadership and PlanningProcess data worksheet; Business Operations ManualISO 9001 StandardDocuments and communicationto the organizationSupporting DocumentsQP-001 Document Control; QP-002 Control of Records;Records: QMS Scope, Organization Chart, Quality Policy, Quality Objectives, Plan to meet objectives, Planned changes to QMSClause Directly Applicable RequirementsInformation:Objective EvidenceIssuesInternal and External Issues4.1NeedsNeeds of Relevant Interested Parties4.2ScopeScope4.3Process DocumentsQMS Processes4.4Leadership Commitment5.1Quality PolicyPolicy5.2Responsibilities andRoles and Responsibilities5.3Authorities assignedRisk / OpportunitiesRisks and Opportunities6.1Spreadsheet, actionsto address6.2Quality Objectives (documented)Quality Objectives6.2.2,6.3Action plan to achieve Q objectives’Planning changes to the QMSAction items,QMS changes4.47.37.58.1ProcessesAwarenessDocumented informationControl of planned changes – includingconsequences and adverse effectsProcess documentsProcess Measure:Top Line SalesGoal: Budget metJan: YFeb: YMar: NApr: NDocuments, recordsAudit Notes:InterviewedQuality Indicators: Print Date: 1/18/18GoalJan17Feb17Mar17Apr173
Intro to the Core ToolsAPQP FMEA MSA SPC PPAP
ContentsIntro to the Core Tools Chapter 1 – APQP Chapter 2 – FMEA Chapter 3 – MSA Chapter 4 – SPC Chapter 5 –PPAPChapter Objectives:-Brief overview of Advanced Product Quality PlanningApplication of APQP?Stages of APQP?How does APQP links to the Standard?Benefits of APQP?2
APQPAPQP stands for Advanced Product Quality Planning. it is how a new or revision to an existing product isdesigned, developed, manufactured and controlledAPQP is. A process, not an event Structured methodology Assures customer satisfactionThe output of APQP is. Is the Continual Improvement of Operation (formerly“Product Realization”)3
Application Existing Products After product launch, process monitoring and review is used as a catalyst for continual improvement to prevent defects as a means for enforcing plant-floor discipline forcontrolling characteristic variation New Products For new products, the existing process monitoring andreview knowledge base acts as an input to the Product Development process brings the operator into the initial phases of product andprocess design Transfer Products Moving product manufacturing from one site to another4
Outputs of Each Phase are Inputsto Subsequent PhasesDetermine Customer Expectations; ability of your Company to meetrequirements, plan the program; provide inputs to development processes.Conduct DFMEA, create and test prototypes; finalizefeatures, function and Special Characteristics; DVP&Rand manufacturing feasibility.Design manufacturing process: ProcessFlow, PFMEA, Control Plan, W.I.’s; determineprocess controls, methods for monitoring &recording results.Verify and Validate product & process; production trial run,capability studies, engineering tests, PPAP, PSW and Project SignOff.Management Review, monitor and measure manufacturing processes & product;manage continual improvement.5
6
APQP Benefits Resources are directed towardcustomer satisfaction Early identification of requiredchanges Changes close to or afterproduct launch are avoided Process can successfullyaccommodate unavoidablechanges A quality product is providedon time at the lowest cost Basis for continualimprovement7
Refer to APQPManual for PhaseDescriptionsAPQP includes.-Manufacturing ProcessDesign & Review-cannot be excludedfrom the QualityManagement System8
Management ReviewInputs (partial list) Results of auditsCustomer feedbackProcess performance and product conformityAPQP status Measurements at specific stagesStatus of preventive and corrective actionsFollow-up actions from previous management reviewsPlanned changes that could affect the QualityManagement SystemRecommendations for improvementAnalysis of actual and potential field-failures and theirimpact on quality, safety or the environment9
ContentsIntroduction to the Core Tools Chapter 1 – APQPChapter 2 – FMEAChapter 3 – MSAChapter 4 – SPCChapter 5 –PPAPChapter Objectives:-Brief overview of Failure Mode & Effects AnalysisDesign FMEA?Process FMEA?Keys to Success?How does FMEA link to the Standard?10
FMEA Definitions - DFMEA/PFMEADesign Failure Mode and Effects Analysis Analytical technique used by designresponsible engineer/team Ensures that potential failure modesand associated cause/mechanisms areconsidered and addressed A Standardized method of identifying,evaluating, and prioritizing risks fromdesign causes To prevent the “customer” fromexperiencing failure modes Design for manufacture and assemblyProcess Failure Mode and Effects Analysis Analytical technique typically led byprocess responsible engineer Intent: to ensure that potential failuremodes and their causes are consideredand addressed Manufacturing, assembly, engineeringteam summarizes items that could gowrong as a process is developed. Itformalizes the mental discipline thatshould be used in planning anymanufacturing process. Meet product expectations11
Program Effectiveness12
Process FMEA development step in AdvancedProduct Quality Planning13
FMEA- Design/ProcessLinkages to IATF 16949:2016 8.3.3.3– Multidisciplinary Approach8.3.5.1 – Design and Development Outputs8.3.3.3 – Special Characteristics8.3.5.2(i)– Control Plan-Mfg. Process DesignCSR – PPAP, APQP and FMEAs14
Keys to FMEA team success Support from managementScope not too largeObjectives well definedObjectives considered relevant and significantA measurable objective for effectiveness- identifiedTeam right-sized for the taskTime allotment for analysis and improvementEffective trainingActivity integrated with organization’s developmentprocess Input information and data are available15
ContentsIntro to the Core Tools Chapter 1 – APQP Chapter 2 – FMEA Chapter 3 – MSA Chapter 4 – SPC Chapter 5 –PPAPChapter Objectives:-Brief overview of Measurement Systems AnalysisWhat is a Measurement System?Application of MSA?What is measurement uncertainty?How to implement a good Measurement System?16
What is a Measurement System?A measurement system is the collection of instrumentsor gages, standards, operations, methods, fixtures,software, personnel, environment and assumptions usedto quantify a unit of measure or fix assessment to afeature characteristic being measured. It is the completeprocess used to obtain measurement.17
What is MSA? Measurement System Analysis (MSA) MSA establishes a uniform method to understand whatmeasurement system analysis is The objective of MSA is to qualify the measurement system foruse in manufacturing The measurement system is verified to determine itsstatistical properties, and the use of them inconformance with accepted standards, our needs andcustomers’ requirements.18
Application of MSA19
Measurement System AnalysisTo evaluate a measurement system, determine if: It has adequate resolution It is statically stable over time Statistical properties are consistent over theexpected range and are acceptable for processanalysis of control The sum of all the variables is an acceptable level ofmeasurement uncertainty.20
Measurement System Analysis (cont’d)Uncertainty of Measurement The range within which the true value of acharacteristic is estimated to reside Can be expressed as statistical distribution of aseries of measurements, standard deviations,probability, percentages, error as the differencebetween actual value minus the true value as apoint on a control chart or diagram, etc.21
Measurement knowledgeto be obtained How large is the measurement error? What are the sources of measurement error? Is the measurement system stable over time? Is the measurement system capable for the study? How can the measurement system be improved?22
Implementing GoodMeasurement Systems Identify all inspection, measuring and test equipment(IMT) and show calibration status Ascertain accuracy and precision of IMT Conduct variation studies of IMT (MSA) Determine validity of previous results when IMT is foundout of calibration23
Implementing GoodMeasurement Systems(cont’d) Establish comprehensive procedures for handling,preservation, cleaning, maintenance and storage of allIMT Records of calibration shall include employee gages Use all criteria (requirements) of MSA24
PPAP 4th editionMSA Requirements 2.2.8 MEASUREMENT SYSTEM ANALYSIS shall includebias, linearity, stability and Gage R&R studies for all newor modified gages, test and measuring equipment.25
Practical SenseMSA Requirements What is in the Control Plan? Does it cover Process and Product Parameters? Does it cover IMT from incoming to outgoing shipments? Does it cover end of line and requirements fordownstream activities? Does it cover lab equipment used for testing and analysis? Does it cover IMT for characterization and validationtesting?26
ContentsIntro to the Core Tools Chapter 1 – APQPChapter 2 – FMEAChapter 3 – MSAChapter 4 – SPCChapter 5 –PPAPChapter Objectives:-Brief overview of Statistical Process ControlWhat is variation?What is stability?What are causes of variation?Capability vs. Stability?What are some tools?How does SPC link to the Standard?27
SPCAcronym for Statistical Process Control Primarily deals with analyzing variation to improveprocess performance in any area or process Focuses on prevention not detectionFundamental issues Understanding of common vs. special cases ofvariation Understand the concept of process capability vs.process control Measurement system must be under control (MSA)28
Stability When the variation is due to common causes only,the process is said to be stableA stable process has predictable variationA stable process is also called a process in “Control”Control Charts are used to illustrate stability29
SPC – Variation A process is in control (stable) if: There is no variation in the location of the center of thedistribution of output over time There is no variation in measures of the dispersion (spread)of the distribution of output over time A process is out-of-control (unstable) if: There is variation in the location of the center of thedistribution of output over time There is variation in measure of the dispersion (spread) ofthe distribution of output over time30
Causes of Variation- ExamplesCommon Causes of Variation Typical traffic on the road Typical play in the slides of a machine Human attentiveness Variation in dimensions in a manufactured batchSpecial Causes of Variation Accident on the road Excess play in a slide, due to wear, over time Illness Variation due to change in a batch or supplier31
SPC – Causes of Variation Common-cause variation is always present in a process;a process is considered stable and predictable whenlimited to this variation Special-cause variation is caused by unusual or externalevents not related to the common causes of variation,making the process unpredictable and unstable32
Capability vs. Stability When a process is out of control, the QMS must firststabilize the process Capability: how effectively a process performs Capability is a term used in reference to a stable process33
Improve Capability The first job of a quality specialist is to identify andeliminate special causes of variation Once the process becomes stable, the next step is toidentify the causes of inherent variation andeliminate them. Improvement in capability can be fulfilled only whenthe process is stable34
Some statistical tools used? X-bar, R-chartsCapability (Cpk, Ppk)Run chartsCause & Effect AnalysisAffinityHistogramParetoScatter Diagram35
Linkages to ISO/IATF 16949:2016 9.1.1.2 Identification of Statistical Tools9.1.1.3 Knowledge of Basic Statistical Concepts8.5.1.1 Control Plan7.1.5.1.1 Measurement System AnalysisCustomer Specific RequirementsCore Tools: APQP, PPAP, SPC, MSA36
ContentsIntro to the Core Tools Chapter 1 – APQP Chapter 2 – FMEA Chapter 3 – MSA Chapter 4 – SPC Chapter 5 –PPAPChapter Objectives:-Brief overview of Production Part Approval ProcessWhat is PPAP?What is the purpose of PPAP?When is PPAP required?Notification?Submission?37
Production Part Approval Process(PPAP)Definition: a standardized method developed for useby suppliers to automotive customers to submit approvals fornew or changed parts to their customers prior to fulfillingproduction orders.PPAP Manual 4th edition defines these requirements.Purpose:-To determine
9.2 Internal Audit X 9.3 Management Review X 10 Improvement 10.1 General X 10.2 Corrective Action X 10.3 Continual Improvement X Process. Print Date: 1/18/18 3 Process Name Documents Defining the Process Process Inputs Process Outputs Leadership and Planning Process data worksheet; Business Operations Manual ISO 9001 Standard Documents and communication to the organization Supporting