Transcription
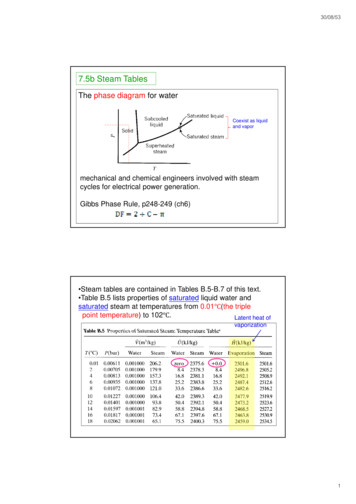
30/08/537.5b Steam TablesThe phase diagram for waterCoexist as liquidand vapormechanical and chemical engineers involved with steamcycles for electrical power generation.Gibbs Phase Rule, p248-249 (ch6) Steam tables are contained in Tables B.5-B.7 of this text. Table B.5 lists properties of saturated liquid water andsaturated steam at temperatures from 0.01Ԩ(the triplepoint temperature) to 102Ԩ.Latent heat ofvaporization1
30/08/53 Determine the vapor pressure, specific internal energy,and specific enthalpy of saturated steam at 25Ԩ.Soln: From Table B.5, T 25 saturated steamP * 0.0317bar , Vˆ 43.4m 3 /kgUˆ 2409.9kJ/kg, Hˆ 2547.3kJ/kgอยากได ไอน้าํ อิ่มตัวที่25C? Show that water at 300Ԩand 5 bar is superheated steamand determine its specific volume, specific internal energyand specific enthalpy relative to liquid water at the triplepoint.2
30/08/53 Show that Û or Ĥ for superheated steam dependstrongly on temperature and relatively slightly onpressure.Example 7.5-3 Energy Balance on a Steam TurbineSteam at 10 bar absolute with 190Ԩ of superheat is fed to aturbine at a rate m 2000 kg/h. The turbine operation isadiabatic, and the effluent is saturated steam at 1 bar.Calculate the work output of the turbine in kilowatts,neglecting kinetic and potential energy changeschanges.P 10bar190oC of superheatm& 2000kg / hturbineP 1barsaturated steamTurbine expansion process3
30/08/53ΔH& ΔE& K ΔE& P Q& W& sQ& 0 (adiabatic )ΔE& 0, ΔE& 0 kPW& s ΔH& m& ( Hˆ out Hˆ in )Table B.7 Properties of Superheated 04
30/08/53 From steam table, the steam at 10 bar is saturated at180oC, so that the inlet steam temperature is 180oC 190oC 370oC. Interpolating in the same table,Hˆ (10bar ,350o C ) 3159kJ / kggHˆ (10bar ,400oC ) 3264kJ / kgถ า temp เพิ่มขึ้น 50C ค า enthalpy จะเพิม่ ขึ้น 105 kJ/kgถ า temp เพิ่มขึ้น 20C ค า enthalpy จะเพิม่ ขึ้น (105x20)/50 42 kJ/kgดังนั้น ที่ 370C ค า enthalpy 3159 42 3201 kJ/kg Hˆ (10bar ,370oC ) 3201kJ / kgP 10bar190oC of superheatHˆ (10bar ,370o C ) 3201kJ / kgturbineP 1barsaturated steamHˆ (1bar , sat ) 2675.4kJ / kgW& s ΔH& m& ( Hˆ out Hˆ in )2000 kgkJ (2675.4 - 3201)kg3600 s 292 kW -The turbine delivers 292 kW of work to its surroundings.5
30/08/537.6 ENERGY BALANCE PROCEDURESExample 7.6-3 Simultaneous Material and EnergyBalancesSaturated steam at 1 atm is discharged from a turbine at arate of 1150 kg/h. Superheated steam at 300Ԩ and 1 atm isneeded as a feed to a heat exchanger; to produce it, theturbine discharge stream is mixed with superheated steamavailable from a second source at 400Ԩ and 1 atm. Themixing input operates adiabatically. Calculate the amount ofsteam at 300Ԩ produced, and the required volumetric flowrate of the 400Ԩ steam.(To the heat exchanger)(Turbine discharge)1150 kg H 2O (v) / hm& 2 kg H 2O(v) / h1atm, saturated (100o C )1atm, 300 o Cmixerm& 1 kg H 2O(v) / h1atm, 400o CThere are two unknown quantities in this process –m& 1 and m& 2 and only one permissible material balance.The material and energy balances must therefore besolved simultaneously to determine the two flow rates.6
30/08/53Turbine discharge1150 kg H 2O(v) / hm& 2 kg H 2O(v) / ho1atm, 300o CHˆ 3074kJ / kg1atm, saturated (100 C )Hˆ 2676kJ / kgmixerm& 1 kg H 2O (v) / h1atm, 400o CHˆ 3278kJ / kgCheck steam tableEnergy balanceΔH& ΔE& K ΔE& P Q& W& s7
30/08/53Mass balance on Water1150kg m& 1 m& 2hEnergy balanceΔH& ΔE& K ΔE& P Q& W& sQ& 0 (process is adiabatic) W& s 0 (no moving parts)ΔE& K 0, ΔE& P 0 (assumption)ΔH& 0ΔH& m& i Hˆ i m& i Hˆ i 0outlet1150inletkJkgkJkJ 2676 m& 1 (3278 ) m& 2 (3074 )hkgkgkgMass balance on Water1150Energy balance115050kg m& 1 m& 2hkgkJkJkJ 2676676 m& 1 (3327878 ) m& 2 (3073074 )hkgkgkgSolving equations 1 and 2 simultaneously yieldsm& 1 2240kg / hm& 2 3390kg / hFrom TableFT bl B.7,B 7 theh specificifi volumeloff steam at 400oCand 1atm is 3.11 m3/kg. The volumetric flow rate ofthis stream is therefore2240kg / h 3.11m3 / kg 6980m3 / h8
30/08/537.7 MECHANICAL ENERGY BALANCES In chemical process units such as reactors, distillationcolumns, evaporators, and heat exchanger, shaft workandd kineticki ti andd potentialt ti l energy changesht d tto betendbnegligible compared with heat flows and internal energyand enthalpy changes. Energy balances usually takethe form Q ΔU (closed system) or Q ΔH (open system). Operations involve the flow of fluids to, from, and between tanks, reservoirs, wells, process units, transportation depots and waste discharges, heat flows andinternal energy changes are secondary in importanceto kinetic and potential energy changes and shaft work. For an open system with one input and one output streamAssume the process fluid is a single incompressible fluid(for example, a liquid) so that Vˆ Vˆ Vˆ and Vˆ 1inoutρ&&&&&ΔH ΔE K ΔE P Q WsΔU& PΔV& V&ΔP 1 m& Δu 2 m& gΔz Q& W&2sW&ΔU&ΔPQ& 12 Δu 2 gΔz sm&m&m&(m& / V ) ΔU& Q& W&ΔP 1 2 2 Δu gΔz sρm& m& m&W&Δu 2 gΔz Fˆ sρm&2ΔPThe mechanicalenergy balanceequation9
30/08/53 A simplified form of the mechanical energy balanceequation is obtained for frictionless process ( Fˆ 0 )and no shaft work is performed ( W& s 0 ).W& sΔu 2ˆ gΔz F 2ρm&ΔP ΔPΔu 2 gΔz 0ρ2Bernoulli equationExample 7.7-1 The Bernoulli EquationWater flows through the system shown here at a rateof 20 L/min. Estimate the pressure required at point (1)if friction losses are negligible.10
30/08/53Soln : The Bernoulli equationΔPΔu 2 gΔz 02ρP2 P1ρ1 (u22 u12 ) g ( z 2 z1 ) 02The velocities arel1m3 1 miniu1 V& / A1 20 / π (0.25 10 2 ) 2 17.0m / smin 1000l 60 sl1m3 1 min&u2 V / A2 20 / π (0.5 10 2 ) 2 4.24m / smin 1000l 60 sP2 P1ρ1 (u22 u12 ) g ( z 2 z1 ) 021.013 105 P1 1 (4.24 2 17 2 ) 9.8 50 0100021.013 105 P1 354.51000 P1 4.56 105 N / m 2 4.5atm11
30/08/53Example 7.7-3 Hydraulic Power GenerationWater flows from an elevated reservoir through a conduitto a turbine at a lower level and out of the turbine througha similar conduit. At a point 100 m above the turbine thepressure is 207 kPa, and at a point 3 m below the turbinethe pressure is 124 kPa. What must the water flow ratebe if the turbine output is 1.00 MW?W&Δu 2 gΔz Fˆ sρm&2ΔP W& s gΔz ρm&ΔPW& s 1.0 106 3ΔP gΔz (124 207) 10 9.8 ( 103)ρ1000 915 kg/sm& 12
Steam tables are contained in Tables B.5-B.7 of this text. Table B.5 lists properties of saturated liquid water and . Steam at 10 bar absolute with 190 (of superheat is fed to a turbine at a rate m 2000 kg/h. The turbine operation is adiabatic, and the effluent is saturated steam at 1 bar. Calculate the work output of the turbine in kilowatts, neglecting kinetic and potential energy .