Transcription
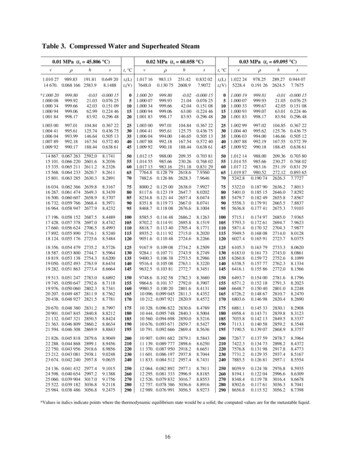
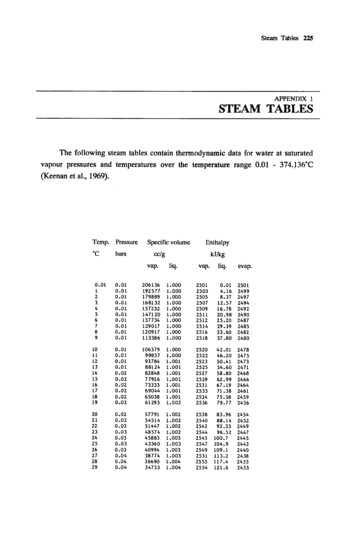
Steam Tables 225APPENDIX 1STEAM TABLESThe following steam tables contain thennodynamic data for water at saturatedvapour pressures and temperatures over the temperature range 0.01 - 374.J36 C(Keenan et aI., 1969).Temp.Pressure·CbarsSpecific 440243824352433
226 Geothermal fluidsTemp.Pressure·CbarsSpecific .0081.0091.0091.0091.0101.0101.0ll1. 29934073281316030442934
Steam Tables 227Temp. Pressure·CbarsSpecific 33.223.323.413.51668.5 ' 5216321602157215421512148
228 Geothennal FluidsTemp.Pressure·CbarsSpecific 119681964
Steam Tables 229Temp.Pressure·CbarsSpecific 6.1984.5883.0181.4779.9678.4977 1
230 Geothermal FluidsTemp. Pressure·cbarsSpecific 125225325425525625725825939.7340.4041.0941. 8429029129229329429529629729829974.3675.4576.5577 113671405139713901382137413661372
Steam Tables 231Temp. Pressure·CbarsSpecific 8.0417.7417.4517 0331332333334335336337338339128.4130.1131. 73.4175.5177 887.5027.3 7.1 68716961704171317221731174117 51893878863847830814796778760741
232 Geothermal fluidsTemp. Pressure·CbarsSpecific 338258910
Atomic Weights 233APPENDIX 2ATOMIC WEIGHTSElementAtomic AtomicSymbol number IodineIridiumIronKryptonLanthanumLawrencium. YZnZrAtomic Atomicnumber 0393040Numbers in parentheses indicate mass number of most stable known 83.85238.0350.942131.30173.0488.90565.3791.22
References 235REFERENCESAggett, J. and Aspell, A.c., 1976. The determination of arsenic (III) and total arsenicby atomic-absorption spectroscopy. Analyst, 101,341-347.APHA, 1989. Standard methods for the examination of water and waste water, 17thedition. America Public Health Association, American Water WorksAssociation, Water Pollution Control Federation, Washington, 1624pp.APHA, 1991. Supplement to the 17th edition of Standard methods for theexamination of water and waste water. America Public Health Association,American Water Works Association, Water Pollution Control Federation,Washington, 15Opp.Aredes, S. and Nicholson, K., 1990. Ammonia in soils: A new technique ingeothermal exploration. Geotherm. Res. Council Trans., 14,1371-1376.Amason, B., 1977a. The hydrogen-water isotope applied to geothermal areas inIceland. Geothermics, 5, 75-80.Amason, B., 1977b. Hydrothermal systems in Iceland traced by deuterium.Geothermics, 5, 125-151.Amorsson, S., 1983. Chemical equilibria in Icelandic geothermal systems.Implications for chemical geothermometry investigations. Geothermics, 12,119-128.Arnorsson, S., 1985. The use of mixing models and chemical geothermometrs forestimating underground temperatures in geothermal systems. J. Volcano!.Geotherm. Res., 23, 299-335.Amorsson, S., 1989. Deposition of calcium carbonate minerals from geothermalwaters - theoretical considerations. Geothermics, 18, 33-39.Amorsson, S. and Gunnlaugsson, E., 1985. New gas geothermometers for geothermalexploration - Calibration and application. Geochim. Cosmochim. Acta, 49,1307-1325.Amorsson, S., Sigurdsson, S. and Svavarsson, H., 1982. The chemistry of geothermalwaters in Iceland. I. Calculation of aqueous speciation from O·C to 370·C.Geochim. Cosmochim. Acta, 46,1513-1532.Amorsson, S., Gunnlaugsson, E. and Svavarsson, H., 1983. The chemistry ofgeothermal waters in Iceland. II. Mineral equilibria and independent variablescontrolling water compositions. Geochim. Cosmochim. Acta, 47, 547-566.
236 Geothennal FluidsASTM, 1992. Annual Book of ASTM Standards. Sections 11.01, 11.02, Water.American Society for Testing Materials, Philadelphia, USA.Aubert, M. and Baubron, J-C., 1988. Identification of a hidden thermal fissure in avolcanic terrain using a combination of hydrothermal convection indicators andsoil-atmosphere analysis. J. Volcano\. Geotherm. Res., 35, 217-225.Ballantyne, 1M. and Moore, J.N., 1988. Arsenic geochemistry in geothermalsystems. Geochim. Cosmochim. Acta, 52, 475-483.Banwart, W.L., Tabatabai, M.A. and Bremner, J.M., 1972. Determination ofammonia in soil extracts and water samples by an ammonia electrode. Comm.Soil Sci. and Plant Analysis, 3, 449-458.Benjamin, T., Charles, R. and Vidale, R., 1983. Thermodynamic parameters andexperimental data for the Na-K-Ca geothermometer. J. Volcano\. Geotherm.Res., 15, 167-186.Berger, B.R. and Bethke, P.M. (eds), 1985. Geology and geochemistry of epithermalsystems. Reviews in Economic Geology, 2, Society of Economic Geologists,298pp.Bergquist, L.F., 1979. Helium: An exploration tool for geothermal sites. Geotherm.Res. Council Trans., 3, 59-60.Bischoff, J.L., Radtke, A.S. and Rosenbauer, R.l, 1981. Hydrothermal alteration ofgreywacke by brine and seawater: roles of alteration and chlorite complexing onmetal solubilities at 200·C and 350 C. Econ. Geol., 79, 659-676.Bogie, I., Lawless, IV. and Pornuevo, lB., 1987. Kaipohan: an apparentlynonthermal manifestation of hydrothermal systems in the Philippines. IVolcano\. Geotherm. Res., 31, 281-292.Bottinga, Y., 1969. Calculated fractionation factors for carbon and hydrogen isotopeexchange in the system calcite-carbon dioxide-graphite-methane-hydrogenwater vapour. Geochim. Cosmochim. Acta, 33, 49-64.Browne, P.R.L., 1978. Hydrothermal alteration in active geothermal fields. Ann.Rev. Earth Planet. Sci., 6, 229-250.Browne, P.R.L., 1979. Minimum age of the Kawerau geothermal field, North Island,New Zealand. I Volcano\. Geotherm. Res., 6, 213-215.Browne, P.R.L., 1987. Hydrothermal alteration processes and their recognition. Precongress workshop on mineralisation and volcanicity. Pacific Rim Congress,Queensland University, 98-149.Browne, P.R.L. and Ellis, A.J., 1970. The Ohaki-Broadlands geothermal area, NewZealand: Mineralogy and related geochemistry. Am. J. Sci., 269, 97-131.
References 237Chemerys, J.e., 1983. Comparison of analytical methods for the determination ofsilica in geothermal water. J. Volcano\. Geotherm. Res., 16,57-63.Chiodini, G. and Cioni, R., 1989. Gas geobarometry for hydrothermal systems and itsapplication to some Italian geothermal areas. Appl. Geochem., 4, 465-472.Christian, G.D. and O'Reilly, IE. (eds), 1986. Instrumental analysis. Allyn andBacon, Boston, 933pp.Cox, M.E., 1980. Ground radon survey of a geothermal area in Hawaii. Geophys.Res. Lett., 7, 4, 283-286.Craig, H., 1953. The geochemistry of stable carbon isotopes. Geochim. Cosmochim.Acta, 3, 53-92.Craig, H., 1957. Isotopic standards for carbon and oxygen and correction factors formass spectrometric analysis of carbon dioxide. Geochim. Cosmochim. Acta,12, 133-149.Craig, H., 1961. Isotopic variations in meteoric waters. Science, 133, 1702-1703.Craig, H., 1963. The isotopic geochemistry of water and carbon in geothermal areas.In: Tongiorgi, E. (ed.), Nuclear geology in geothermal areas, Spoleto, 1963.Consiglio Nazional delle Ricerche, Laboratorio di Geologia Nucleare, Pias, 1753.Craig, H., Boato, G. and White, D.E., 1956. Isotopic geochemistry of thermal waters.National Acad. Sci. National Research Council Publication 400, 29-38.D' Amore, F. and Nuti, S., 1977. Notes on the chemistry of geothermal gases.Geothermics, 6, 39-45.D' Amore, F. and Panichi,e., 1980.Evaluation of deep temperatures of hydrothermalsystems by a new gas-geothermometer. Geochim. Cosmochim. Acta, 44, 549556.D' Amore, F. and Panichi, C., 1987. Geochemistry in geothermal exploration. In:Economides, M. and Ungemach, P. (eds) Applied geothermics, Wiley & Sons,New York, 69-89.D' Amore, F. and Truesdell, A.H., 1984. Helium in the larderello geothermal fluid.Geothermics, 13,227-239.D' Amore, F., Celati. R., Ferrara, G.C. and Panichi. C., 1977. Secondary changes inthe chemical and isotopic composition of the geothermal fluids in Larderellofield. Geothermics, 5, 153-163.D' Amore, F., FancelIi, R., Saracco, L. and Truesdell, A.H., 1987. Gasgeothermometry based on CO content - Applications in Italian geothermalfields. Proc. 12th Workshop on Geothermal Reservoir Engineering, StandordUniversity, USA, 247-252.
238 Geothermal FluidsDeitz, W.A, 1967. I. Gas Chromatog., 5, 68-70. Cited by Ellis et aI., 1968.Del Moro, A, Puxeddu, M., Radicati di Brozolo, F. and Villa, I.M., 1982. Rb-Sr andK-Ar ages on minerals at temperatures of 300·C-400·C from deep wells in theLarderello geothermal field (Italy). Contrib. Mineral. Petrol., 81, 340-349.DesMarais, D.I., Donchin, I.H., Nehring, N.L. and Truesdell, AH., 1981. Molecularcarbon isotopic evidence for the origin of geothermal hydrocarbons. Nature,292,826.DoE/NWC, 1978. Mercury in waters, effluents and sludges by f1ameless atomicabsorption spectrophotometry. Methods for the examination of waters andassociated materials. The Standing Committee of Analysts, The Department ofthe EnvironmentlNational Water Council, HMSa, London, 35pp.DoE/NWC,1980. Boron in waters, effluents, sewage and some solids 1980.Methods for the examination of waters and associated materials. The StandingCommittee of Analysts, The Department of the EnvironmentlNational WaterCouncil, HMSa, London, 35pp.Duchi, V., Minissale, AA, and Prati, F., 1987b. Chemical composition of thermalsprings, cold springs, streams and gas vents in the Mt Amiata geothermal region(Tuscany, Italy). I. Volcano\. Geotherm. Res., 31, 321-332.Duchi, V., Minissale, AA, artino, S. and Roman, L., 1987a. Geothermalprospecting by geochemical methods on natural gas and water discharges in theVulsini Mts volcanic district (Central Intaly). Geothermics, 16, 147-157.Ellis, AI., 1962. Interpretation of gas analysis from the Wairakei hydrothermal area.N.Z. 1. Sci., 5, 434-452.Ellis, AI., 1968. Natural hydrothermal systems and experimental hot water/rockinteraction: reaction with NaCI solutions and trace metal extraction. Geochim.Cosmochim. Acta, 32, 1356-1363.Ellis, AI., 1969. Present-day hydrothermal systems and mineral deposition. Proc.9th Commonwealth mining and metal I. congress (mining and petroleumseetion), Instn Min. Metal\., London, 1-30.Ellis, AI., 1970. Quantitative interpretation of chemical characteristics ofhydrothermal systems. Geothermics, Special Issue 2, 1,516-528.Ellis, AI., 1977. Chemical and isotopic techniques in geothermal investigations.Geothermics, 5, 3-12.Ellis, AI., 1979. Chemical geothermometry in geothermal systems. Geothermics,25, 219-226.Ellis, AI. and Golding, R.M., 1963. The solubility of carbon dioxide above lOOT inwater and in sodium chloride solutions. Am. I. Sci., 261, 47-60.
References 239Ellis, AJ. and Mahon. W.AJ., 1964. Natural hydrothermal systems and experimentalhot-water/rock interactions. Geochim. Cosmochim. Acta, 28, 1323-1357.Ellis, AJ.and Mahon. W.AJ., 1967. Natural hydrothermal systems and experimentalhot-water/rock interactions (part 11). Geochim. Cosmochim. Acta, 31, 519-538.Ellis, AJ.and Mahon. W.Al, 1977. Chemistry and geothermal systems. AcademicPress, New York, 392pp.Ellis, AJ.and Wilson, S.H., 1960. The geochemistry of alkali metal ions in theWairakei hydrothermal system. N.Z. J. GeoI. Geophys., 3, 593-617.Ellis, AJ., Mahon, W.AJ. and Ritchie, lA, 1968. Methods of collection andanalysis of geothermal fluids. Second edition. Report CD21 03, ChemistryDivision, DSIR, New Zealand, 51pp.EPRI, 1985. A theory on trace arsenic in geothermal fluids. Research Project 15256, final report AP-4214. Electric Power Research Institute, California, USAEPRI, 1986. A theory on boron in geothermal fluids. Research Project 1525-6, finalreport AP-4670. Electric Power Research Institute, California, USAEPRI, 1987. A theory on mercury in geothermal fluids. Research Project 1525-6,final report AP-AP-5111. Electric Power Research Institute, California, USA.Finlayson, J.B., 1970. The collection and analysis of volcanic and hydrothermalgases. Geothermics, Special Issue, 2 (2), 1344-1354.Fletcher, W.F., Hoffman, S.l, Mehrtens, M.B., Sinclair, AJ. and Thomson, 1.,1986.Exploration geochemistry: Design and interpretation of soil surveys. Reviewsin Economic Geology, 3, Society of Economic Geologists, 180pp.Fouillac, C. and Michard, G., 1981. Sodium/lithium ratio in water applied togeothermometry of geothermal reservoirs. Geothermics, 10, 55-70.Fournier, R.O., 1977. Chemical Geothermometers and mixing models for geothermalsystems. Geothermics, 5, 41-50.Fournier, R.O., 1979a. A revised equation for the NalK geothermometer. Geoth.Res. Council Trans., 3, 221-224.Fournier, R.O., 1979b. Geochemical and hydrological considerations and the use ofenthalpy-chloride diagrams in the prediction of underground conditions in hotspring systems. I Volcano!. Geotherm. Res., 5, 1-16.Fournier, R.O., 1981. Application of water geochemistry to geothermal explorationand reservoir engineering. In: Rybach, L. and Muffler, L.IP., Geothermalsystems: Principals and case histories. Wiley, Chichester, 109-143.Fournier, R.O., 1983. A method of calculating quartz solubilities in aqueous sodiumchloride solutions. Geochim. Cosmochim. Acta, 47, 579-586.
240 G(othennal FluidsFournier, R.O., 1985. The behaviour of silica in hydrothermal solutions. In: Berger,B.R. and Bethke, P.M. (eds) Geology and geochemistry of epithermal systems.Reviews in Economic Geology, 2, Society of Economic qeologists, 45-61.Fournier, R.O., 1989a. Lectures on geochemical interpetation of hydrothermalwaters. UNU Geothermal Training Programme, Reykjavik, Iceland, Report 10,73pp.Fournier, R.O., 1989b. Geochemistry and dynamics of the Yellowstone NationalPark hydrothermal system. Ann. Rev. Earth Planet Sci. 17, 13-53.Fournier, R.O., 1990. The interpretation of Na-K-Mg relations in geothermal aters.Geoth. Res. Council Trans., 14, 1421-1425.Fournier, R.O.and Marshall, W.L., 1983. Calculation of amorphous silica solubilitiesat 25·C to 300·C and apparent cation hydration numbers in aqueous saltsolutions using the concept of effective density of water. Geochim.Cosmochim. Acta, 47, 587-596.Fournier, R.O. and Potter, R.W., 1979. Magnesium correction to the Na-K-Cachemical geothermometer. Geochim. Cosmochim. Acta, 43, 1543-1550.Fournier, R.O. and Potter, R.W., 1982a. An equation correlating the solubility ofquartz in water from 25·C to 9OO·C at pressures up to 10,000 bars. Geochim.Cosmochim. Acta, 46,1969-1974.Fournier, R.O. and Potter, R.W., 1982b. A revised and expanded silica (quartz)geothermometer. Geoth. Res. Council Bull., November, 3-12.Fournier, R.O. and Rowe, J.J., 1966. estimation of underground temperatures fromthe silica content of water from hot springs and wet-steam wells. Amer. J. Sci.,264, 685-697.Fournier, R.O. and Truesdell, A.H., 1973. An empirical Na-K-Ca geothermometerfor natural waters. Geochim. Cosmochim. Acta, 37,1255-1275.Fournier, R.O. and Truesdell, A.H., 1974. Geochemical indicators of subsurfacetemperature - Part 2, estimation of temperature and fraction of hot water mixedwith cold water. J. Res. U.S. Geol. Survey, 2, 263-270.Fournier, R.O., Sorey, M.L., Mariner, R.H. and Truesdell, A.H., 1979. Chemical andisotopic prediction of aquifer temperatures in the geothermal system at LongValley, California. J. Volcanol. Geotherm. Res., 5,17-34.Fournier, R.O., White, D.E. and Truesdell, A.H., 1974. Geochemical indicators ofsubsurface temperature - Part 1, basic assumptions. J. Res. U.S. Geol. Survey,2,259-261.
References 241Friedman, I. and O'Neill, IR., 1977. Compilation of stable isotope fractionationfactors of geochemical interest. In: Fleischer, M. (ed.), Data of geochemistry,U.S. Geol. Survey Prof. Paper, 440-KI(. 12pp.Fritz, IS. and Schenk, G.H., 1979. Quantitative analytical chemistry. Fourth edition.A1lyn and Bacon, Boston, 661pp.Fritz, P. and Fontes, J.Ch., 1980. Introduction. In: Fritz, P. and Fontes, J.Ch. (eds),Handbook of environmental isotope geochemistry, Volume 1, The terrestrialenvironment A. Elsevier, Amsterdam, 1-19.Ghomshel, M.M., Croft. S.A.S. and Stauder. J.J. 1986. Geochemical evidence ofchemical equilibria in the south Meager Creek geothermal system, BritishColuinbia, Canada. Geothermics. 15. 49-61.Giggenbach. W.F., 1971. Isotopic composition of waters ofthe Broadlandsgeothermal field. N.Z. J. Sci., 14,959-970.Giggenbach, W.F., 1975. A simple method for the collection and analysis of volcanicgas samples. Bull. Volcanol. 39,132-145.Giggenbach, W.F., 1978. The isotopic composition of waters from the EI Tatiogeothermal field. northern Chile. Geochim. Cosmochim. Acta. 42. 979-988.Giggenbach. W.F. 1980. Geothermal gas equilibria. Geochim. Cosmochim. Acta.44.2021-2032.Giggenbach. W.F. 1981. Geothermal mineral equilibria. Geochim. Cosmochim.Acta. 45, 393-410.Giggenbach. W.F., 1982. Carbon-13 exchange between CO2 and CF4 undergeothermal conditions. Geochim. Cosmochim. Acta, 46, 159-165.Giggenbach. W.F., 1984. Mass transfer in hydrothermal alteration systems - Aconceptual approach. Geochim. Cosmochim. Acta, 48, 2693-2711.Giggenbach. W.F., 1987. Redox process governing the chemistry of fumarolic gasdischarges from White Island. New Zealand. Appl. Geochem., 2. 143-161.Giggenbach, W.F., 1988. Geothermal solute equilibria. Derivation of Na-K-Mg-Cageoindicators. Geochim. Cosmochim. Acta, 52, 2749-2765.Giggenbach, W.F. and Goguel, R.L., 1989. Collection and analysis of geothermaland volcanic water and gas discharges. Fourth Edition. Report CD2401,Chemistry Division, DSIR, New Zealand.Giggenbach. W.F. and Stewart, M.K., 1982. Processes controlling the isotopiccomposition of steam and water discharges from steam vents and steam-heatedp
Steam Tables 225 APPENDIX 1 STEAM TABLES The following steam tables contain thennodynamic data for water at saturated vapour pressures and temperatures over the temperature range 0.01 - 374.J36 C (Keenan et aI., 1969). Temp. Pressure Specific vol