Transcription
Index to Tables in SI UnitsTable A-1Table A-2Table A-3Table A-4Table A-5Table A-6Table A-7Table A-8Table A-9Table A-10Table A-11Table A-12Table A-13Table A-14Table A-15Table A-16Table A-17Table A-18Table A-19Table A-20Table A-21Table A-22Table A-23Table A-24Table A-25Table A-26Table A-27Atomic or Molecular Weights and Critical Properties of Selected Elements and Compounds 926Properties of Saturated Water (Liquid–Vapor): Temperature Table 927Properties of Saturated Water (Liquid–Vapor): Pressure Table 929Properties of Superheated Water Vapor 931Properties of Compressed Liquid Water 935Properties of Saturated Water (Solid–Vapor): Temperature Table 936Properties of Saturated Refrigerant 22 (Liquid–Vapor): Temperature Table 937Properties of Saturated Refrigerant 22 (Liquid–Vapor): Pressure Table 938Properties of Superheated Refrigerant 22 Vapor 939Properties of Saturated Refrigerant 134a (Liquid–Vapor): Temperature Table 943Properties of Saturated Refrigerant 134a (Liquid–Vapor): Pressure Table 944Properties of Superheated Refrigerant 134a Vapor 945Properties of Saturated Ammonia (Liquid–Vapor): Temperature Table 948Properties of Saturated Ammonia (Liquid–Vapor): Pressure Table 949Properties of Superheated Ammonia Vapor 950Properties of Saturated Propane (Liquid–Vapor): Temperature Table 954Properties of Saturated Propane (Liquid–Vapor): Pressure Table 955Properties of Superheated Propane Vapor 956Properties of Selected Solids and Liquids: cp, r, and k. 960Ideal Gas Specific Heats of Some Common Gases 961Variation of cp with Temperature for Selected Ideal Gases 962Ideal Gas Properties of Air 963Ideal Gas Properties of Selected Gases 965Constants for the van der Waals, Redlich–Kwong, and Benedict–Webb–RubinEquations of State 969Thermochemical Properties of Selected Substances at 298 K and 1 atm 970Standard Molar Chemical Exergy, ech (kJ/kmol), of Selected Substances at 298 K and p0 971Logarithms to the Base 10 of the Equilibrium Constant K 972925
926Tables in SI UnitsTable A-1TABLE A-1Atomic or Molecular Weights and Critical Properties of SelectedElements and micalFormulaAcetyleneAir 00.2740.274CarbonCarbon dioxideCarbon rigerant 12Refrigerant 249.80.2760.2780.267Refrigerant 134aSulfur .340.778.7220.90.2600.2680.233Zc ⴝSources: Adapted from International Critical Tables and L. C. Nelson and E. F. Obert, Generalized CompressibilityCharts, Chem. Eng., 61: 203 (1954).Sources for Tables A-2 through A-18.Tables A-2 through A-6 are extracted from J. H. Keenan, F. G. Keyes, P. G. Hill, and J. G. Moore, Steam Tables,Wiley, New York, 1969.Tables A-7 through A-9 are calculated based on equations from A. Kamei and S. W. Beyerlein, “A FundamentalEquation for Chlorodifluoromethane (R-22),” Fluid Phase Equilibria, Vol. 80, No. 11, 1992, pp. 71–86.Tables A-10 through A-12 are calculated based on equations from D. P. Wilson and R. S. Basu, “ThermodynamicProperties of a New Stratospherically Safe Working Fluid — Refrigerant 134a,” ASHRAE Trans., Vol. 94, Pt. 2,1988, pp. 2095–2118.Tables A-13 through A-15 are calculated based on equations from L. Haar and J. S. Gallagher, “ThermodynamicProperties of Ammonia,” J. Phys. Chem. Reference Data, Vol. 7, 1978, pp. 635–792.Tables A-16 through A-18 are calculated based on B. A. Younglove and J. F. Ely, “Thermophysical Properties ofFluids. II. Methane, Ethane, Propane, Isobutane and Normal Butane,” J. Phys. Chem. Ref. Data, Vol. 16, No. 4, 1987,pp. 577–598.
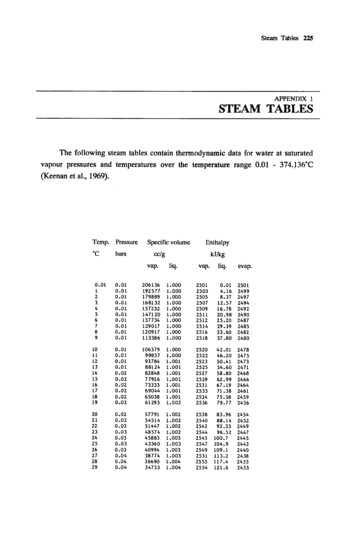
Tables in SI Units927TABLE A-2Properties of Saturated Water (Liquid–Vapor): Temperature TableSpecific Volumem3/kgInternal EnergykJ/kgTemp.8CPress.barSat.Liquidvf 3 343536384045Sat.LiquidhfEntropykJ/kg ? 78.35318.33368.29508.25708.16483536384045vf 5 (table ons:Pressure ConvPaM10. rba12 10 kPa
928Tables in SI UnitsTABLE A-2(Continued)H2Oersions:Pressure ConvPaM1 bar 0.12 10 kPaSpecific Volumem3/kgInternal EnergykJ/kgTemp.8CPress.barSat.Liquidvf 3 85397.88EnthalpykJ/kgSat.LiquidhfEntropykJ/kg ? 20340360374.14300320340360374.14vf 5 (table value)/1000
Tables in SI Units929TABLE A-3Properties of Saturated Water (Liquid–Vapor): Pressure TableSpecific Volumem3/kgTemp.8CSat.Liquidvf 3 at.LiquidhfEntropykJ/kg ? 25.61415.552770.080.090.0100.110.vf 5 (table barSat.VaporvgInternal EnergykJ/kgPress.barH2Oersions:Pressure ConvPaM10. rba12 10 kPa
930Tables in SI UnitsTABLE A-3H2Oersions:Pressure ConvPaM1 bar 0.12 10 kPa(Continued)Specific Volumem3/kgTemp.8CSat.Liquidvf 3 55Press.barSat.VaporvgInternal EnergykJ/kgEnthalpykJ/kgEntropykJ/kg ? .190.200.220.9vf 5 (table value)/1000
Tables in SI Units931TABLE A-4Properties of Superheated Water Vaporvm3/kgukJ/kghkJ/kgskJ/kg ? Kvm3/kgp 5 0.06 bar 5 0.006 MPa(Tsat 5 36.168C)ukJ/kghkJ/kgskJ/kg ? Kp 5 0.35 bar 5 0.035 MPa(Tsat 5 14909.3194p 5 0.70 bar 5 0.07 MPa(Tsat 5 89.958C)p 5 1.0 bar 5 0.10 MPa(Tsat 5 032.63131.63278.23361.43488.18.54358.66368.8342p 5 1.5 bar 5 0.15 MPa(Tsat 5 111.378C)p 5 3.0 bar 5 0.30 MPa(Tsat 5 03703.28.15388.32518.5892Pressure Conversions:1 bar 0.1 MPa 10 2 kPaH2OT8C
932Tablews in SI UnitsTABLE A-4(Continued)H2OT8Cersions:Pressure ConvPaM10.1 bar 2 10 kPavm3/kgukJ/kghkJ/kgskJ/kg ? Kvm3/kgp 5 5.0 bar 5 0.50 MPa(Tsat 5 151.868C)ukJ/kghkJ/kgskJ/kg ? Kp 5 7.0 bar 5 0.70 MPa(Tsat 5 24.87.92998.19568.4391p 5 10.0 bar 5 1.0 MPa(Tsat 5 179.918C)p 5 15.0 bar 5 1.5 MPa(Tsat 5 83.87.68057.83857.9391p 5 20.0 bar 5 2.0 MPa(Tsat 5 212.428C)p 5 30.0 bar 5 3.0 MPa(Tsat 5 11.77.50857.61067.7571
Tables in SI Units933TABLE A-4(Continued)vm3/kgukJ/kghkJ/kgskJ/kg ? Kvm3/kgukJ/kgp 5 40 bar 5 4.0 MPa(Tsat 5 250.48C)hkJ/kgskJ/kg ? Kp 5 60 bar 5 6.0 MPa(Tsat 5 13528.33752.63894.13989.27.27317.42347.5190p 5 80 bar 5 8.0 MPa(Tsat 5 295.068C)p 5 100 bar 5 10.0 MPa(Tsat 5 34.73512.13723.73870.53968.17.01317.16877.2670p 5 120 bar 5 12.0 MPa(Tsat 5 324.758C)p 5 140 bar 5 14.0 MPa(Tsat 5 e Conversions:1 bar 0.1 MPa 10 2 kPaH2OT8C
934Tables in SI UnitsTABLE A-4(Continued)H2OT8Cersions:Pressure ConvPaM10.1 bar 2 10 kPavm3/kgukJ/kghkJ/kgskJ/kg ? Kvm3/kgukJ/kgp 5 160 bar 5 16.0 MPa(Tsat 5 347.448C)hkJ/kgskJ/kg ? Kp 5 180 bar 5 18.0 MPa(Tsat 5 0.024833396.33478.03821.53925.06.85806.9623p 5 200 bar 5 20.0 MPa(Tsat 5 365.818C)p 5 240 bar 5 24.0 600.016333548.03762.74015.14285.16.79667.0372p 5 280 bar 5 28.0 MPap 5 320 bar 5 32.0 MPa
Tables in SI Units935TABLE A-5Properties of Compressed Liquid Waterv 3 103m3/kgukJ/kghkJ/kgskJ/kg ? Kv 3 103m3/kgp 5 25 bar 5 2.5 MPa(Tsat 5 223.998C)ukJ/kghkJ/kgskJ/kg ? Kp 5 50 bar 5 5.0 MPa(Tsat 5 .1938.41147.8853.9944.41154.22.32552.51282.9202p 5 75 bar 5 7.5 MPa(Tsat 5 290.598C)p 5 100 bar 5 10.0 MPa(Tsat 5 6936.21124.41282.0945.11134.01292.2p 5 150 bar 5 15.0 MPa(Tsat 5 342.248C)934.11121.11393.0p 5 200 bar 5 20.0 MPa(Tsat 5 35962.036929.91114.61316.61585.6p 5 250 bar 5 25 64.60412.08834.51296.6v 5 (table 20714.0139p 5 300 bar 5 30.0 3021.330482.17164.04410.78831.41287.9v 5 (table 1.28442.28933.1741Pressure Conversions:1 bar 0.1 MPa 10 2 kPaH2OT8C
6218220222224226228230232234236238240v 5 (table i 3 .1206.3241.7Sat.VaporvgSpecific .82710.2Subl.uigInternal orugProperties of Saturated Water (Solid–Vapor): Temperature TableTemp.8CPressure Conversions:1 bar 0.1 MPa 102 kPaTABLE ropykJ/kg ? 489.4149.1569.1579.219Sat.Vaporsg
Tables in SI Units937TABLE A-7Properties of Saturated Refrigerant 22 (Liquid–Vapor): Temperature TableSpecific Volumem3/kgInternal EnergykJ/kgEnthalpykJ/kgEntropykJ/kg ? KTemp.8CPress.barSat.Liquidvf 3 6030.8455323640455060vf 5 (table value)/1000R-22ersions:Pressure ConvPaM10. rba12 10 kPa
938Tables in SI UnitsTABLE A-8R-22ersions:Pressure ConvPaM10. rba12 10 kPaProperties of Saturated Refrigerant 22 (Liquid–Vapor): Pressure TableSpecific Volumem3/kgInternal EnergykJ/kgEnthalpykJ/kgEntropykJ/kg ? KPress.barTemp.8CSat.Liquidvf 3 4790.75210.75610.1
Sources for Tables A-2 through A-18. Tables A-2 through A-6 are extracted from J. H. Keenan, F. G. Keyes, P. G. Hill, and J. G. Moore, Steam Tables, Wleyi , New York, 1969. Tables A-